Nitrosation introduces a nitroso group (-NO) into a molecule, typically affecting amines to form nitrosamines, while nitration adds a nitro group (-NO2) mainly to aromatic compounds, altering their chemical properties and reactivity. Understanding these distinct electrophilic substitution reactions helps you optimize conditions for targeted chemical synthesis and safety considerations.
Table of Comparison
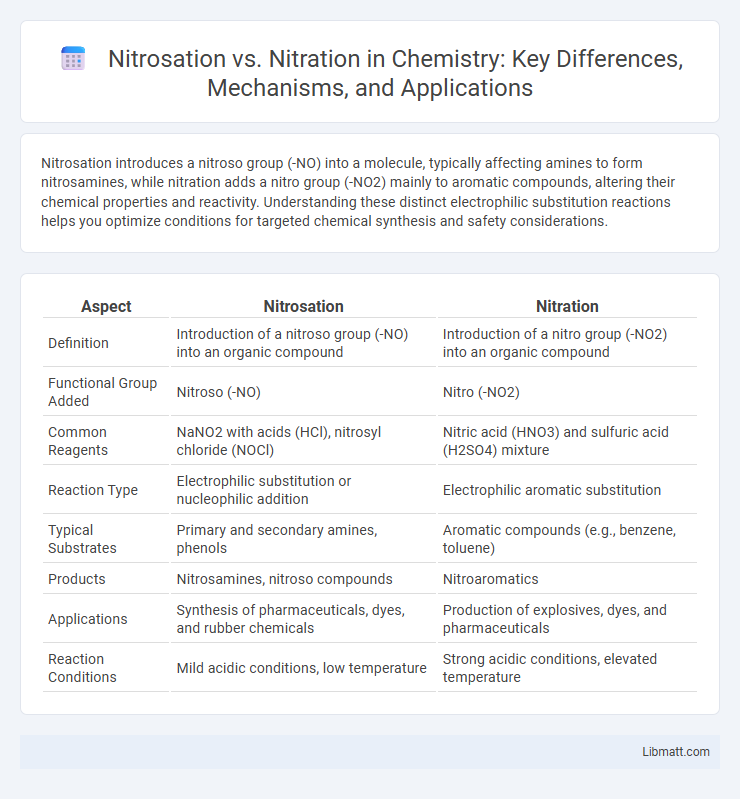
| Aspect | Nitrosation | Nitration |
|---|---|---|
| Definition | Introduction of a nitroso group (-NO) into an organic compound | Introduction of a nitro group (-NO2) into an organic compound |
| Functional Group Added | Nitroso (-NO) | Nitro (-NO2) |
| Common Reagents | NaNO2 with acids (HCl), nitrosyl chloride (NOCl) | Nitric acid (HNO3) and sulfuric acid (H2SO4) mixture |
| Reaction Type | Electrophilic substitution or nucleophilic addition | Electrophilic aromatic substitution |
| Typical Substrates | Primary and secondary amines, phenols | Aromatic compounds (e.g., benzene, toluene) |
| Products | Nitrosamines, nitroso compounds | Nitroaromatics |
| Applications | Synthesis of pharmaceuticals, dyes, and rubber chemicals | Production of explosives, dyes, and pharmaceuticals |
| Reaction Conditions | Mild acidic conditions, low temperature | Strong acidic conditions, elevated temperature |
Introduction to Nitrosation and Nitration
Nitrosation involves the introduction of a nitroso group (-NO) into organic compounds, commonly occurring in amines and leading to the formation of nitrosamines. Nitration is a key electrophilic aromatic substitution reaction where a nitro group (-NO2) is introduced, typically using a mixture of nitric and sulfuric acids, and is crucial in the synthesis of explosives, dyes, and pharmaceuticals. Both processes significantly alter molecular reactivity and properties, with nitrosation often implicated in mutagenesis and nitration used for functionalizing aromatic rings.
Fundamental Differences: Nitrosation vs Nitration
Nitrosation involves the introduction of a nitroso group (-NO) into an organic compound, typically through electrophilic attack by nitrosonium ions (NO+), whereas nitration refers to the substitution of a nitro group (-NO2) usually via electrophilic aromatic substitution using nitrating agents such as a mixture of nitric acid and sulfuric acid. Nitrosation reactions commonly occur under milder conditions and can affect amines and phenols, forming nitroso derivatives, while nitration is primarily employed to introduce nitro groups into aromatic rings, significantly altering their electronic properties and reactivity. The fundamental difference lies in the nature of the functional group introduced and the mechanistic pathways, with nitrosation involving nitroso group addition and nitration involving nitro group substitution.
Mechanisms of Nitrosation Reactions
Nitrosation reactions involve the transfer of a nitroso group (NO) to a nucleophile, typically occurring under acidic conditions where nitrous acid (HNO2) generates the nitrosonium ion (NO+), the key electrophile. The mechanism proceeds through the electrophilic attack of NO+ on amines, leading to the formation of nitrosamines via a diazonium intermediate or directly by nitrosylation of the nitrogen atom. This contrasts with nitration, which introduces a nitro group (NO2) via electrophilic aromatic substitution involving the nitronium ion (NO2+), highlighting distinct reactive species and pathways in these nitrogen-based functionalization processes.
Mechanisms of Nitration Reactions
Nitration reactions primarily occur through electrophilic aromatic substitution, where a nitronium ion (NO2+) acts as the electrophile attacking the aromatic ring. This process involves the generation of the nitronium ion typically from a mixture of nitric acid and sulfuric acid, which facilitates the substitution of a hydrogen atom with a nitro group (NO2). You can leverage this mechanism to understand reactivity patterns and optimize nitration conditions in synthetic organic chemistry.
Key Reagents and Conditions
Nitrosation typically involves nitrous acid (HNO2) or sodium nitrite (NaNO2) under acidic conditions, often using hydrochloric acid (HCl) as a catalyst to generate the nitrosating agent. Nitration requires a mixture of concentrated nitric acid (HNO3) and sulfuric acid (H2SO4), where sulfuric acid acts as a protonating agent to form the nitronium ion (NO2+), the active electrophile. Your choice of reaction depends on these distinct reagents and conditions to target either nitroso or nitro functional groups efficiently.
Biological and Industrial Relevance
Nitrosation involves the addition of a nitroso group (-NO) to amines or thiols, playing a crucial role in cellular signaling and the formation of nitrosamines linked to cancer risk in biological systems, while nitration introduces a nitro group (-NO2) primarily affecting aromatic compounds, impacting protein function through tyrosine nitration in inflammation and neurodegenerative diseases. Industrially, nitrosation is essential for synthesizing pharmaceuticals and rubber accelerators, whereas nitration is widely used in producing explosives, dyes, and polymers. Understanding the distinct mechanisms and effects of nitrosation versus nitration can help you optimize processes in drug development and industrial chemical manufacturing.
Common Substrates in Nitrosation and Nitration
Nitrosation commonly targets secondary amines, phenols, and thiols, converting them into nitrosamines, nitrosophenols, and nitrosothiols, respectively, while nitration primarily involves aromatic compounds such as benzene, toluene, and phenol, introducing nitro groups into their ring structures. The electrophilic substitution in nitration typically uses nitric acid and sulfuric acid to generate the nitronium ion (NO2+), which attacks electron-rich aromatic rings. Nitrosation mechanisms often involve nitrous acid (HNO2) as the nitrosating agent, facilitating the transfer of the nitrosonium ion (NO+) to nucleophilic sites in molecules.
Safety and Environmental Impact
Nitrosation reactions often involve nitrosating agents that can release toxic nitrosamines, posing significant health risks and environmental concerns due to their carcinogenic properties. Nitration processes typically use strong acids like nitric and sulfuric acid, which require careful handling to prevent harmful emissions and chemical spills affecting the environment. Understanding the safety protocols and environmental impact of these reactions is crucial for protecting your health and minimizing ecological damage during chemical synthesis.
Analytical Methods for Detection
Analytical methods for detecting nitrosation primarily involve spectrophotometry, gas chromatography-mass spectrometry (GC-MS), and high-performance liquid chromatography (HPLC) to identify nitrosamines and their derivatives. Nitration detection often utilizes techniques such as UV-Vis spectroscopy, Fourier-transform infrared spectroscopy (FTIR), and nuclear magnetic resonance (NMR) to analyze nitro compounds formed. Your choice of method depends on the specificity, sensitivity, and matrix of the sample requiring precise quantification of nitrosation or nitration products.
Summary: Choosing Between Nitrosation and Nitration
Nitrosation involves introducing a nitroso group (-NO) into organic compounds, typically targeting amines to form nitrosamines, whereas nitration adds a nitro group (-NO2) usually to aromatic rings, producing nitroarenes. Selecting between nitrosation and nitration depends on the desired chemical functionality and the substrate's reactivity; nitrosation is preferred for modifying amines, while nitration is ideal for electrophilic aromatic substitution. Reaction conditions, such as acid medium and temperature, also influence the choice, with nitration often requiring stronger acids like sulfuric acid and nitrosation proceeding under milder conditions.
Nitrosation vs nitration Infographic
 libmatt.com
libmatt.com