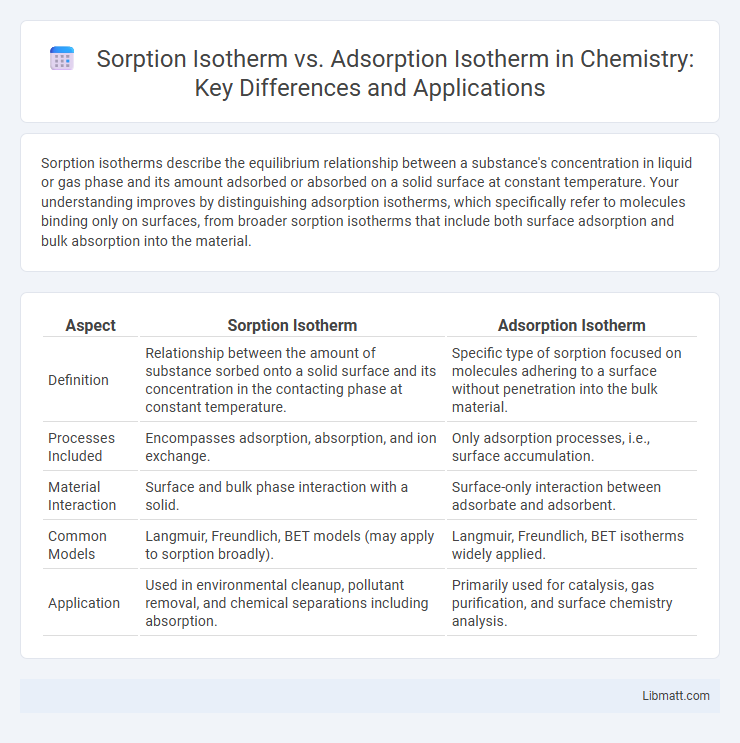
Sorption isotherms describe the equilibrium relationship between a substance's concentration in liquid or gas phase and its amount adsorbed or absorbed on a solid surface at constant temperature. Your understanding improves by distinguishing adsorption isotherms, which specifically refer to molecules binding only on surfaces, from broader sorption isotherms that include both surface adsorption and bulk absorption into the material.
Table of Comparison
| Aspect | Sorption Isotherm | Adsorption Isotherm |
|---|---|---|
| Definition | Relationship between the amount of substance sorbed onto a solid surface and its concentration in the contacting phase at constant temperature. | Specific type of sorption focused on molecules adhering to a surface without penetration into the bulk material. |
| Processes Included | Encompasses adsorption, absorption, and ion exchange. | Only adsorption processes, i.e., surface accumulation. |
| Material Interaction | Surface and bulk phase interaction with a solid. | Surface-only interaction between adsorbate and adsorbent. |
| Common Models | Langmuir, Freundlich, BET models (may apply to sorption broadly). | Langmuir, Freundlich, BET isotherms widely applied. |
| Application | Used in environmental cleanup, pollutant removal, and chemical separations including absorption. | Primarily used for catalysis, gas purification, and surface chemistry analysis. |
Introduction to Sorption and Adsorption Isotherms
Sorption isotherms describe the equilibrium relationship between the amount of a substance sorbed onto a solid phase and its concentration in the contacting phase at constant temperature. Adsorption isotherms, a subset of sorption isotherms, specifically quantify the uptake of gases or solutes on solid surfaces, characterizing adsorption capacity and surface interactions. Key models such as Langmuir, Freundlich, and BET isotherms provide critical insights into adsorption mechanisms and surface heterogeneity in various scientific and industrial applications.
Fundamental Concepts: Sorption vs Adsorption
Sorption isotherms represent the equilibrium relationship between the amount of a substance absorbed or adsorbed and its concentration at constant temperature, encompassing both adsorption on surfaces and absorption within materials. Adsorption isotherms specifically describe the accumulation of molecules on the surface of solids or liquids, highlighting surface interactions and surface area effects. Understanding the differences in fundamental mechanisms between sorption and adsorption is essential for applications in material science, environmental engineering, and chemical processing.
Types of Sorption Isotherms
Sorption isotherms describe the relationship between the amount of substance sorbed and its concentration at constant temperature, classifying into several types based on shape: Type I (Langmuir) indicates monolayer adsorption on microporous materials, Type II and III represent multilayer adsorption on non-porous or macroporous surfaces, Type IV shows capillary condensation in mesoporous materials, and Type V is similar to Type III but with stronger adsorbate-adsorbent interactions. Adsorption isotherms specifically refer to the physical adsorption of gases or vapors on solid surfaces, often modeled using these same isotherm types to understand surface heterogeneity and pore distribution. Your choice of isotherm type depends on the sorption mechanism and material properties critical for applications like catalysis, environmental remediation, or material design.
Types of Adsorption Isotherms
Adsorption isotherms describe the relationship between the amount of adsorbate on the adsorbent surface and its concentration at constant temperature, with key types including Langmuir, Freundlich, BET, and Temkin isotherms. Sorption isotherms encompass both adsorption and absorption processes, illustrating how substances partition between phases. Understanding these adsorption isotherms helps you optimize material selection and surface interaction in applications like catalysis, environmental remediation, and gas storage.
Key Differences Between Sorption and Adsorption Isotherms
Sorption isotherms describe the relationship between the amount of a substance retained on a solid phase and its concentration in a liquid or gas phase at equilibrium, encompassing both adsorption and absorption processes. Adsorption isotherms specifically detail the equilibrium distribution of molecules only on the surface of a solid, without penetration into the bulk phase. Key differences include sorption isotherms accounting for both surface adherence and internal uptake, while adsorption isotherms focus exclusively on surface phenomena, influencing modeling approaches and interpretation in environmental science and material engineering.
Mathematical Models of Sorption and Adsorption
Mathematical models of sorption and adsorption isotherms describe the relationship between the amount of substance adsorbed or sorbed and its concentration at equilibrium. Common models for adsorption isotherms include Langmuir and Freundlich, reflecting monolayer and heterogeneous surface adsorption, respectively, while sorption isotherms often incorporate additional mechanisms such as absorption into phases beyond surface retention. Understanding these models helps you accurately predict sorbate behavior in systems involving environmental remediation, material science, and chemical engineering processes.
Factors Influencing Isotherm Behavior
Sorption isotherm behavior is influenced by factors such as temperature, pressure, and the nature of the sorbent and sorbate, affecting the equilibrium between phases. Adsorption isotherms specifically depend on surface area, pore size distribution, and the chemical affinity between the adsorbate and adsorbent, which determine the capacity and adsorptive strength. Understanding these parameters helps you tailor materials for optimized sorption performance in applications like gas storage or wastewater treatment.
Applications of Sorption and Adsorption Isotherms
Sorption and adsorption isotherms are essential for understanding material interactions in environmental science, catalysis, and pharmaceuticals by quantifying how substances adhere or absorb on surfaces under varying conditions. Adsorption isotherms are widely used to design and optimize filtration systems, predict pollutant behavior in water treatment, and enhance catalyst efficiency in chemical reactions. Sorption isotherms provide critical insights for soil moisture retention, pesticide uptake, and drug delivery systems, enabling precise control over the storage and release processes crucial to your applications.
Experimental Methods for Isotherm Measurement
Sorption isotherm measurement involves experimental techniques such as gravimetric analysis and volumetric methods, which quantify the amount of substance sorbed at equilibrium under controlled temperature and pressure. Adsorption isotherm experiments typically use manometric or volumetric setups to determine the adsorption capacity by monitoring gas uptake on solid surfaces. Your choice of experimental method should align with the material properties and the specific type of isotherm--sorption or adsorption--to achieve accurate and reliable results.
Current Trends and Future Perspectives in Isotherm Research
Sorption isotherms and adsorption isotherms continue to be critical in understanding material interactions with gases and liquids, with current trends focusing on advanced modeling techniques such as machine learning integration and high-throughput experimentation. Researchers are increasingly exploring nanoscale sorbents and bio-inspired materials to enhance selectivity and capacity, addressing challenges in environmental remediation and energy storage. Your ability to leverage these innovative approaches will shape future perspectives, driving more accurate predictions and efficient design in isotherm research.
Sorption isotherm vs adsorption isotherm Infographic
 libmatt.com
libmatt.com