Hydrocracking uses hydrogen and a catalyst to break down heavy hydrocarbons into lighter, more valuable products like diesel and jet fuel, offering higher yields and cleaner outputs compared to fluid catalytic cracking (FCC), which primarily relies on a catalyst to crack heavy oils into gasoline and lighter products but produces more sulfur and residues. Your choice depends on desired product quality, feedstock type, and refinery goals, with hydrocracking favored for producing low-sulfur fuels and FCC preferred for maximizing gasoline output.
Table of Comparison
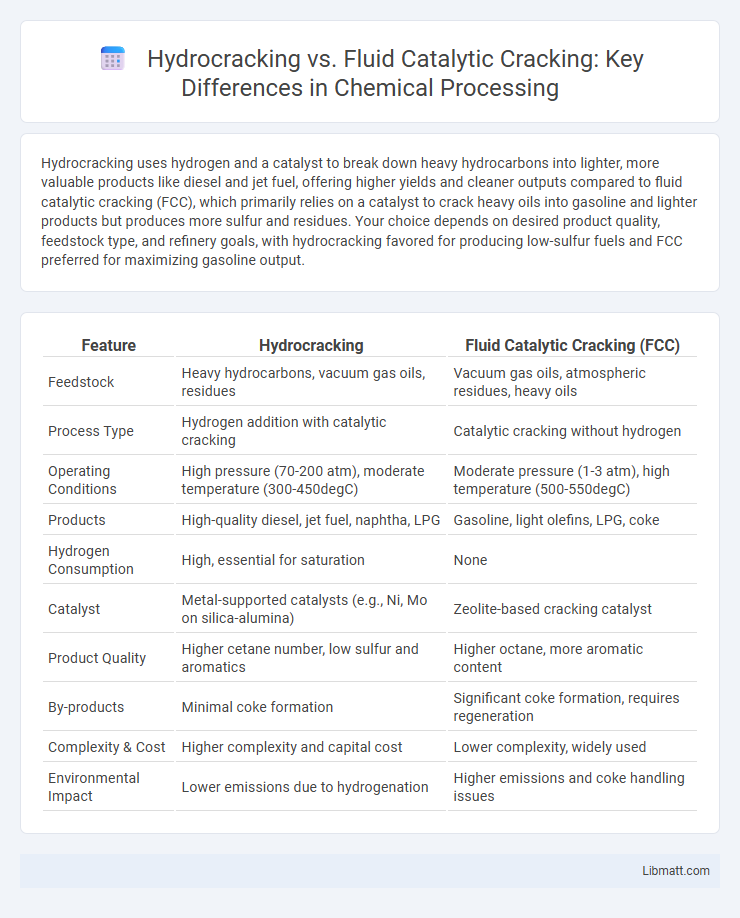
| Feature | Hydrocracking | Fluid Catalytic Cracking (FCC) |
|---|---|---|
| Feedstock | Heavy hydrocarbons, vacuum gas oils, residues | Vacuum gas oils, atmospheric residues, heavy oils |
| Process Type | Hydrogen addition with catalytic cracking | Catalytic cracking without hydrogen |
| Operating Conditions | High pressure (70-200 atm), moderate temperature (300-450degC) | Moderate pressure (1-3 atm), high temperature (500-550degC) |
| Products | High-quality diesel, jet fuel, naphtha, LPG | Gasoline, light olefins, LPG, coke |
| Hydrogen Consumption | High, essential for saturation | None |
| Catalyst | Metal-supported catalysts (e.g., Ni, Mo on silica-alumina) | Zeolite-based cracking catalyst |
| Product Quality | Higher cetane number, low sulfur and aromatics | Higher octane, more aromatic content |
| By-products | Minimal coke formation | Significant coke formation, requires regeneration |
| Complexity & Cost | Higher complexity and capital cost | Lower complexity, widely used |
| Environmental Impact | Lower emissions due to hydrogenation | Higher emissions and coke handling issues |
Introduction to Hydrocracking and Fluid Catalytic Cracking
Hydrocracking and fluid catalytic cracking (FCC) are two critical refining processes that convert heavy petroleum fractions into valuable lighter products such as gasoline, diesel, and jet fuel. Hydrocracking uses hydrogen and a catalyst under high pressure and temperature to break down complex hydrocarbons, improving product yield and quality while reducing sulfur content. Fluid catalytic cracking operates at lower pressures and uses a zeolite catalyst to crack heavy hydrocarbon molecules through a catalytic reaction, producing high-octane gasoline and other light hydrocarbons efficiently.
Basic Principles of Hydrocracking
Hydrocracking involves breaking down heavy hydrocarbons into lighter, more valuable products using high pressure, hydrogen gas, and a catalyst, enabling the removal of sulfur and nitrogen impurities. Fluid catalytic cracking (FCC) operates at lower pressure, relying on a catalyst to crack heavy oil fractions into lighter fuels without hydrogen gas. You can optimize refining efficiency by understanding that hydrocracking offers higher selectivity for producing diesel and jet fuel, while FCC is typically used for gasoline production.
Core Mechanisms of Fluid Catalytic Cracking
Fluid Catalytic Cracking (FCC) operates through catalytic cracking, where heavy hydrocarbons are broken into lighter molecules by catalysts at high temperatures. The core mechanism involves the adsorption of feedstock on a zeolite catalyst surface followed by carbocation formation, chain scission, and hydrogen transfer reactions. This process primarily produces gasoline and olefins, with advantages in catalyst regeneration and high conversion efficiency, distinguishing it from hydrocracking which uses hydrogen and metal catalysts for saturation and cleavage.
Feedstock Requirements and Flexibility
Hydrocracking processes heavier, high-boiling feedstocks such as vacuum gas oils and residual oils, offering greater flexibility by upgrading a wide range of feedstock varieties with varying sulfur and metal contents. Fluid catalytic cracking (FCC) operates primarily on lighter feedstocks like gas oils and atmospheric residues, requiring cleaner inputs due to catalyst sensitivity to contaminants. Your choice depends on feedstock availability and desired product quality, as hydrocracking tolerates heavier and more contaminated feedstocks while FCC demands relatively cleaner charges for efficient conversion.
Operating Conditions: Temperature and Pressure
Hydrocracking operates under high pressure, typically between 70 to 200 atmospheres, and moderate temperatures ranging from 260 to 425degC, enabling the catalytic hydrogenation of heavy hydrocarbons. Fluid catalytic cracking (FCC) functions at much lower pressure, around atmospheric levels, but at significantly higher temperatures, generally between 480 to 550degC, to crack heavy oil feedstocks in the presence of a solid catalyst. Your choice of process depends on feedstock characteristics and desired product quality, as operating conditions directly influence conversion efficiency and product composition.
Product Yields and Quality Comparison
Hydrocracking produces higher yields of high-quality middle distillates such as diesel and jet fuel with lower sulfur and aromatics content, making it ideal for producing cleaner fuels. Fluid catalytic cracking (FCC) primarily yields gasoline and light olefins but generates more by-products like coke and sulfur compounds, resulting in lower diesel quality. Hydrocracking's hydrogen addition process enhances product stability and cetane number, while FCC focuses on maximizing gasoline volume and octane rating.
Catalyst Types and Lifespan
Hydrocracking utilizes bifunctional catalysts combining metal sites such as palladium or nickel with acidic support materials like zeolites, which enables both hydrogenation and cracking reactions, resulting in longer catalyst lifespan due to reduced coke formation. Fluid catalytic cracking (FCC) employs solid acid catalysts primarily made from zeolites, which are highly active but more susceptible to deactivation by coke, requiring frequent regeneration. Your choice between these processes depends on desired product slate and catalyst durability under operational conditions.
Environmental Impacts and Emissions
Hydrocracking produces significantly lower sulfur oxides (SOx), nitrogen oxides (NOx), and particulate emissions compared to fluid catalytic cracking (FCC) due to its hydrogen-rich environment and use of catalysts that promote cleaner fuel production. FCC units release higher amounts of carbon monoxide (CO) and unburned hydrocarbons, contributing to smog and air pollution, whereas hydrocracking generates higher quality fuels with reduced aromatics and contaminants. The lower greenhouse gas emissions and more efficient sulfur removal in hydrocracking make it a preferred choice for meeting stringent environmental regulations in the refining industry.
Economic Considerations and Cost Efficiency
Hydrocracking generally incurs higher operational costs due to expensive catalysts and hydrogen consumption but delivers higher yields of premium diesel and jet fuels, enhancing overall economic value. Fluid catalytic cracking (FCC) offers lower initial investment and catalyst expenses, making it more cost-efficient for gasoline production but yields lower-quality products requiring further upgrading. Refineries balance hydrocracking's upgrading benefits against FCC's cost efficiency based on feedstock quality, product slate goals, and market dynamics to optimize profitability.
Choosing the Optimal Cracking Process
Selecting the best cracking process depends on your desired product output and feedstock characteristics; hydrocracking excels in producing high-quality diesel and jet fuel with lower sulfur content, while fluid catalytic cracking (FCC) is more effective for maximizing gasoline yield from heavier crude fractions. Hydrocracking uses hydrogen under high pressure and temperature with a catalyst to break down complex molecules, resulting in cleaner fuels and higher conversion rates, whereas FCC operates at lower pressure and relies on catalyst regeneration to convert heavy oils into lighter gasoline and olefins. Evaluating your refinery's capacity, economic goals, and environmental regulations are crucial factors in determining whether hydrocracking or FCC meets your operational needs.
Hydrocracking vs fluid catalytic cracking Infographic
 libmatt.com
libmatt.com