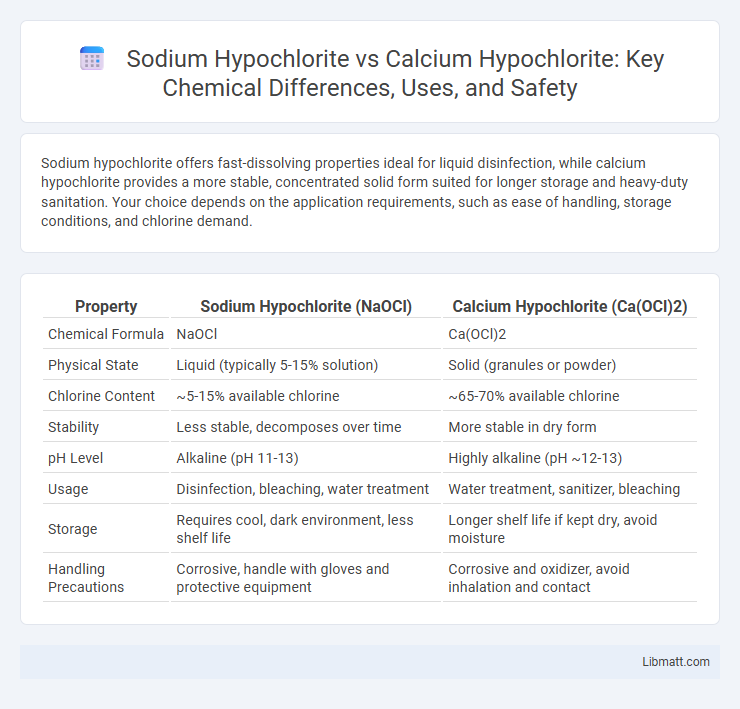
Sodium hypochlorite offers fast-dissolving properties ideal for liquid disinfection, while calcium hypochlorite provides a more stable, concentrated solid form suited for longer storage and heavy-duty sanitation. Your choice depends on the application requirements, such as ease of handling, storage conditions, and chlorine demand.
Table of Comparison
| Property | Sodium Hypochlorite (NaOCl) | Calcium Hypochlorite (Ca(OCl)2) |
|---|---|---|
| Chemical Formula | NaOCl | Ca(OCl)2 |
| Physical State | Liquid (typically 5-15% solution) | Solid (granules or powder) |
| Chlorine Content | ~5-15% available chlorine | ~65-70% available chlorine |
| Stability | Less stable, decomposes over time | More stable in dry form |
| pH Level | Alkaline (pH 11-13) | Highly alkaline (pH ~12-13) |
| Usage | Disinfection, bleaching, water treatment | Water treatment, sanitizer, bleaching |
| Storage | Requires cool, dark environment, less shelf life | Longer shelf life if kept dry, avoid moisture |
| Handling Precautions | Corrosive, handle with gloves and protective equipment | Corrosive and oxidizer, avoid inhalation and contact |
Introduction to Sodium Hypochlorite and Calcium Hypochlorite
Sodium hypochlorite is a widely used liquid chemical compound with the formula NaOCl, commonly employed as a disinfectant and bleaching agent. Calcium hypochlorite, with the chemical formula Ca(ClO)2, is a solid, highly concentrated compound primarily used for water treatment and sanitation purposes. Both compounds release chlorine when dissolved in water, making them effective for sterilization, but calcium hypochlorite offers higher chlorine content and longer shelf life compared to sodium hypochlorite.
Chemical Composition and Structure Comparison
Sodium hypochlorite (NaOCl) is an aqueous solution or solid compound consisting of sodium, oxygen, and chlorine, with a molecular structure featuring a hypochlorite ion bonded to a sodium ion, offering strong oxidizing properties. Calcium hypochlorite (Ca(ClO)2) is a solid compound composed of calcium ions and two hypochlorite ions, arranged in a crystalline lattice that provides a higher available chlorine content per unit weight compared to sodium hypochlorite. The structural difference results in calcium hypochlorite being more stable in solid form and commonly used as a granular disinfectant, whereas sodium hypochlorite is often utilized as a liquid bleach.
Production Methods and Availability
Sodium hypochlorite is produced through the chlorination of sodium hydroxide, typically using a continuous flow process in industrial plants, resulting in a liquid bleach solution widely available for household and commercial use. Calcium hypochlorite is manufactured by reacting lime (calcium hydroxide) with chlorine gas, forming a solid granular or tablet form that offers higher stability and is readily accessible for water treatment and sanitation applications. Both compounds are mass-produced globally, with sodium hypochlorite favored for liquid disinfection needs and calcium hypochlorite preferred for solid chlorine products due to its longer shelf life and ease of transport.
Mechanism of Action as Disinfectants
Sodium hypochlorite and calcium hypochlorite disinfect by releasing hypochlorous acid (HOCl) when dissolved in water, which penetrates microbial cell walls and oxidizes essential cellular components, leading to microbial death. Sodium hypochlorite, commonly found in liquid bleach, provides a fast-acting and readily soluble source of free chlorine, whereas calcium hypochlorite, typically in granular or tablet form, slowly dissolves to maintain prolonged chlorination. Both compounds generate reactive oxidative species that disrupt proteins, lipids, and nucleic acids, but calcium hypochlorite's higher available chlorine content offers longer-lasting disinfection in water treatment applications.
Applications in Water Treatment and Pool Maintenance
Sodium hypochlorite, commonly used in liquid form, is highly effective for disinfecting drinking water and treating wastewater due to its quick dissolution and ease of application. Calcium hypochlorite, available as a solid granule or tablet, offers a higher concentration of available chlorine, making it ideal for pool maintenance and shock treatments where long-lasting chlorine levels are necessary. Both compounds serve as powerful oxidizing agents but differ in stability and usage contexts within water treatment and pool sanitation processes.
Stability, Storage, and Shelf Life
Sodium hypochlorite offers lower stability compared to calcium hypochlorite, as it degrades faster when exposed to light, heat, and air, requiring storage in a cool, dark place with airtight containers. Calcium hypochlorite exhibits higher stability and a longer shelf life, often lasting up to six months or more under proper dry and cool storage conditions. Both chemicals must be handled carefully, but calcium hypochlorite's solid form enhances its durability and reduces the risk of rapid decomposition during storage.
Safety Considerations and Handling Precautions
Sodium hypochlorite requires careful handling due to its corrosive nature and potential to release chlorine gas when mixed with acids, posing respiratory hazards. Calcium hypochlorite is a stronger oxidizer, demanding strict storage away from organic materials and moisture to prevent explosive reactions and fire risks. Proper personal protective equipment (PPE), adequate ventilation, and secure containers are essential safety measures for both chemicals to minimize exposure and accidental releases.
Environmental Impact and Biodegradability
Sodium hypochlorite and calcium hypochlorite differ significantly in environmental impact and biodegradability; sodium hypochlorite breaks down more rapidly in the environment, reducing long-term chlorine residues, while calcium hypochlorite is more stable and can persist longer in aquatic systems, posing higher risks to aquatic life. Calcium hypochlorite often results in higher pH levels and can introduce calcium ions that affect water chemistry, influencing ecosystem health, whereas sodium hypochlorite tends to have a milder effect on water pH. Choosing the appropriate disinfectant for your application involves considering these factors to minimize ecological harm and enhance biodegradability.
Cost Analysis and Economic Considerations
Sodium hypochlorite typically offers a lower upfront cost and easier handling due to its liquid form, making it a cost-effective choice for short-term disinfection needs. Calcium hypochlorite, available as a solid, has a higher concentration of available chlorine, providing greater long-term economic value through reduced storage and transportation expenses. Your decision should weigh these factors alongside application scale and frequency to optimize overall cost-efficiency.
Choosing the Right Hypochlorite: Key Factors
Sodium hypochlorite offers rapid disinfection with high solubility, making it ideal for liquid applications and immediate use, whereas calcium hypochlorite provides a more stable, solid form with a higher chlorine concentration, suitable for long-term storage and slower release. Key factors in choosing between them include application method, desired disinfection speed, stability requirements, and handling safety. Water treatment systems often favor sodium hypochlorite for continuous dosing, while pools benefit from calcium hypochlorite for shock treatments due to its potent chlorine content and ease of transport.
Sodium hypochlorite vs calcium hypochlorite Infographic
 libmatt.com
libmatt.com