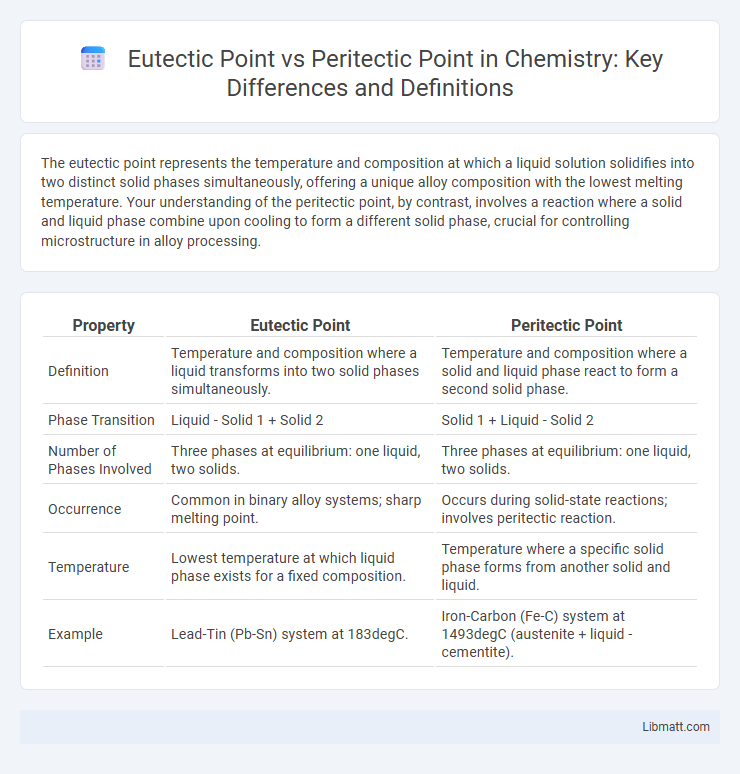
The eutectic point represents the temperature and composition at which a liquid solution solidifies into two distinct solid phases simultaneously, offering a unique alloy composition with the lowest melting temperature. Your understanding of the peritectic point, by contrast, involves a reaction where a solid and liquid phase combine upon cooling to form a different solid phase, crucial for controlling microstructure in alloy processing.
Table of Comparison
| Property | Eutectic Point | Peritectic Point |
|---|---|---|
| Definition | Temperature and composition where a liquid transforms into two solid phases simultaneously. | Temperature and composition where a solid and liquid phase react to form a second solid phase. |
| Phase Transition | Liquid - Solid 1 + Solid 2 | Solid 1 + Liquid - Solid 2 |
| Number of Phases Involved | Three phases at equilibrium: one liquid, two solids. | Three phases at equilibrium: one liquid, two solids. |
| Occurrence | Common in binary alloy systems; sharp melting point. | Occurs during solid-state reactions; involves peritectic reaction. |
| Temperature | Lowest temperature at which liquid phase exists for a fixed composition. | Temperature where a specific solid phase forms from another solid and liquid. |
| Example | Lead-Tin (Pb-Sn) system at 183degC. | Iron-Carbon (Fe-C) system at 1493degC (austenite + liquid - cementite). |
Introduction to Eutectic and Peritectic Points
Eutectic and peritectic points describe distinct phase reactions in alloy systems crucial for understanding microstructure formation during cooling. The eutectic point occurs at a specific composition and temperature where a liquid transforms directly into two solid phases simultaneously, maximizing compositional homogeneity. Your knowledge of these points enables better control of alloy properties by predicting critical temperature-composition relationships in phase diagrams.
Definition of Eutectic Point
The eutectic point is the specific composition and temperature at which a mixture of substances solidifies simultaneously from the liquid phase into two or more solid phases. It represents the lowest temperature at which the liquid phase can exist for that particular alloy system. Understanding the eutectic point is crucial for controlling microstructure and mechanical properties in materials engineering.
Definition of Peritectic Point
The peritectic point is a specific temperature and composition in a phase diagram where a solid and a liquid phase react to form a second solid phase upon cooling. Unlike the eutectic point, where a liquid transforms directly into two solid phases simultaneously, the peritectic reaction involves the transformation of one solid phase and liquid into another solid phase. This key difference influences alloy solidification processes and microstructure development in materials science.
Key Differences Between Eutectic and Peritectic Points
The eutectic point represents a specific temperature and composition where a liquid phase solidifies into two distinct solid phases simultaneously, while the peritectic point involves a reaction where a liquid and one solid phase transform into a different solid phase upon cooling. At the eutectic point, the material transitions directly from liquid to two solids, whereas the peritectic reaction is a solid-phase transformation occurring at a specific temperature and alloy composition. Understanding these key differences enables you to predict phase behavior accurately in alloy systems and optimize material properties accordingly.
Phase Diagrams: Eutectic vs Peritectic Reactions
Eutectic and peritectic points represent critical phase transformations in phase diagrams, with the eutectic point indicating the lowest temperature at which a liquid transforms directly into two solid phases simultaneously. Peritectic points occur when a liquid and one solid phase combine upon cooling to form a second solid phase, typically at a specific temperature and composition. Understanding these reactions helps you interpret complex alloy solidification behaviors and optimize material properties.
Thermodynamic Principles Underlying Eutectic and Peritectic Points
Eutectic and peritectic points represent distinct phase equilibria governed by thermodynamic principles in binary alloy systems. The eutectic point occurs where the liquid phase coexists with two solid phases at the lowest melting temperature, reflecting a minimum in the Gibbs free energy diagram for the system. In contrast, the peritectic point involves a reaction where a solid and liquid phase combine to form a different solid phase upon cooling, characterized by a unique intersection in the phase diagram and a complex Gibbs free energy relationship.
Microstructural Outcomes of Eutectic and Peritectic Reactions
Eutectic points result in a fine, lamellar microstructure formed by the simultaneous crystallization of two solid phases from a liquid at a specific composition and temperature, enhancing mechanical properties like strength and toughness. Peritectic points produce a more complex microstructure due to the solid-liquid-solid reaction, where one solid phase reacts with the liquid to form a second solid phase, often leading to coarser grains and varied phase distributions. Understanding these differences in microstructural outcomes helps you tailor thermal processing techniques to optimize alloy performance.
Real-World Examples of Eutectic and Peritectic Systems
The eutectic point in the lead-tin alloy system enables soldering by melting at a precise 183degC, ensuring reliable electronic connections. The peritectic point in the iron-carbon phase diagram, specifically the transformation of delta ferrite and liquid to austenite near 1495degC, plays a critical role in steel manufacturing processes. Understanding these phase transitions helps you optimize cooling rates and alloy compositions for improved material properties in industrial applications.
Industrial Applications of Eutectic and Peritectic Alloys
Eutectic alloys, known for their sharp melting points and fine microstructures, are widely used in soldering, casting, and thermal interface materials due to their predictable solidification behavior. Peritectic alloys find applications in high-temperature structural components and advanced alloys for aerospace and power generation because of their unique phase formation and transformation characteristics. Understanding the eutectic and peritectic phase diagrams enables precise control of alloy composition, enhancing mechanical properties and manufacturing efficiency in various industrial processes.
Conclusion: Importance in Materials Science
The eutectic point represents the lowest temperature at which a mixture of substances can coexist in liquid and solid phases, critical for controlling alloy solidification and achieving desired microstructures. The peritectic point involves a reaction where a solid and liquid phase transform into a second solid phase, influencing phase stability and transformation pathways in metallic systems. Understanding these phase equilibria enables materials scientists to tailor thermal treatments and compositions, optimizing mechanical properties and performance in advanced materials.
Eutectic point vs peritectic point Infographic
 libmatt.com
libmatt.com