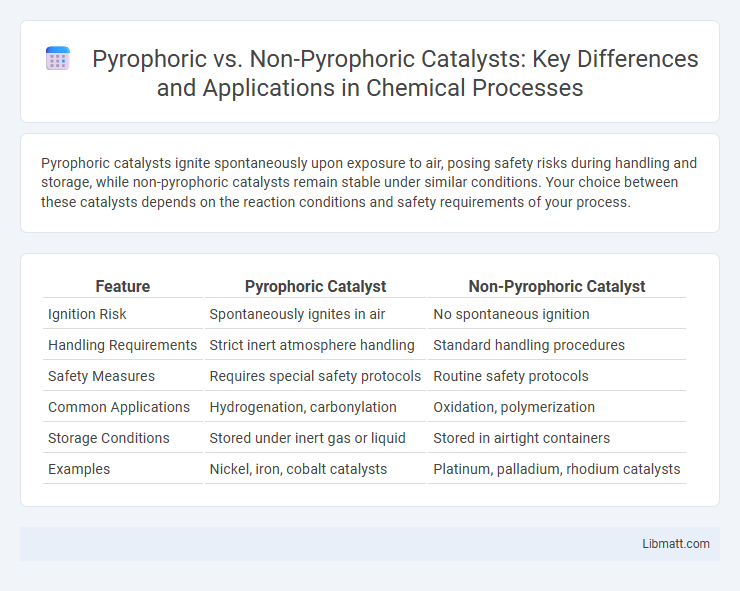
Pyrophoric catalysts ignite spontaneously upon exposure to air, posing safety risks during handling and storage, while non-pyrophoric catalysts remain stable under similar conditions. Your choice between these catalysts depends on the reaction conditions and safety requirements of your process.
Table of Comparison
| Feature | Pyrophoric Catalyst | Non-Pyrophoric Catalyst |
|---|---|---|
| Ignition Risk | Spontaneously ignites in air | No spontaneous ignition |
| Handling Requirements | Strict inert atmosphere handling | Standard handling procedures |
| Safety Measures | Requires special safety protocols | Routine safety protocols |
| Common Applications | Hydrogenation, carbonylation | Oxidation, polymerization |
| Storage Conditions | Stored under inert gas or liquid | Stored in airtight containers |
| Examples | Nickel, iron, cobalt catalysts | Platinum, palladium, rhodium catalysts |
Introduction to Pyrophoric and Non-Pyrophoric Catalysts
Pyrophoric catalysts are substances that ignite spontaneously upon exposure to air due to their high reactivity with oxygen, commonly used in processes requiring highly active catalytic sites. Non-pyrophoric catalysts, in contrast, remain stable and do not ignite in air, making them safer for storage and handling in various industrial applications. Understanding the differences in reactivity and handling between pyrophoric and non-pyrophoric catalysts is essential for selecting appropriate materials in chemical synthesis and catalytic processes.
Defining Pyrophoric Catalysts: Properties and Examples
Pyrophoric catalysts are materials that spontaneously ignite upon exposure to air due to their highly reactive surface properties, such as finely divided metals like iron sulfide and certain alkyl aluminum compounds. These catalysts exhibit high surface energy and extreme sensitivity to oxygen, requiring inert atmosphere handling to prevent hazardous ignition. Non-pyrophoric catalysts, such as supported platinum or palladium on carbon, do not ignite spontaneously and are typically more stable under ambient conditions, offering safer handling during catalytic processes.
What Are Non-Pyrophoric Catalysts? Characteristics and Types
Non-pyrophoric catalysts are materials that do not spontaneously ignite when exposed to air, offering safer handling and storage in industrial processes. These catalysts typically consist of metal oxides, supported metals, or mixed metal compounds that exhibit thermal stability and resistance to oxidation under ambient conditions. Common types include supported platinum or palladium catalysts on silica or alumina, zeolites, and transition metal oxides, which are widely used in hydrogenation, oxidation, and reforming reactions due to their controlled reactivity and durability.
Key Differences Between Pyrophoric and Non-Pyrophoric Catalysts
Pyrophoric catalysts ignite spontaneously in air due to their high reactivity, posing significant handling and storage challenges, while non-pyrophoric catalysts remain stable under normal atmospheric conditions, ensuring safer usage. The key differences include the presence of highly reactive metals or alloys in pyrophoric catalysts that cause rapid oxidation, compared to inert or less reactive constituents in non-pyrophoric catalysts. These distinctions impact catalyst selection for industrial processes, balancing activity, safety, and operational protocols in chemical synthesis and refining applications.
Safety Considerations: Handling and Storage
Pyrophoric catalysts ignite spontaneously upon exposure to air, requiring inert atmosphere handling and specialized sealed containers to ensure safety during storage. Non-pyrophoric catalysts pose less risk and can often be stored under standard conditions but still demand proper labeling and containment to prevent contamination. Your handling protocols should prioritize minimizing air exposure and use appropriate protective equipment to reduce the risk of ignition or hazardous reactions.
Industrial Applications: Pyrophoric vs Non-Pyrophoric Catalysts
Pyrophoric catalysts are extensively utilized in industrial applications that require highly reactive surfaces for processes such as hydrogenation and olefin polymerization, where their ability to ignite spontaneously in air enhances catalytic efficiency but demands stringent safety protocols. Non-pyrophoric catalysts are preferred in large-scale chemical reactors and refinery operations where stability and reduced fire risk are critical, enabling safer handling and longer catalyst lifespans under harsh operational conditions. Industries like petrochemical refining, pharmaceutical synthesis, and specialty chemical production tailor catalyst selection based on the trade-off between activity and stability, optimizing process yields and minimizing downtime due to catalyst deactivation or hazardous incidents.
Advantages and Disadvantages of Pyrophoric Catalysts
Pyrophoric catalysts offer the advantage of high reactivity and rapid activation at lower temperatures, which enhances reaction efficiency and reduces energy consumption. However, their tendency to ignite spontaneously upon exposure to air poses significant handling and safety risks, requiring stringent storage and operational protocols. Non-pyrophoric catalysts, while safer and easier to manage, often require higher activation energies and may result in slower reaction rates compared to pyrophoric catalysts, impacting process efficiency.
Benefits and Limitations of Non-Pyrophoric Catalysts
Non-pyrophoric catalysts offer enhanced safety by eliminating the risk of spontaneous ignition when exposed to air, making them ideal for large-scale industrial applications. These catalysts often exhibit stable performance under ambient conditions, reducing the need for specialized handling and storage equipment. However, their activity and selectivity may be lower compared to pyrophoric catalysts, potentially requiring higher reaction temperatures or longer times to achieve desired conversion rates.
Selection Criteria: Choosing the Right Catalyst for Your Process
Selecting between pyrophoric and non-pyrophoric catalysts hinges on safety requirements, reaction conditions, and operational control. Pyrophoric catalysts offer high activity but require stringent handling protocols due to their spontaneous ignition risk, making them suitable for inert atmosphere processes. Non-pyrophoric catalysts provide safer handling and stability, ideal for processes prioritizing operational safety over catalytic activity.
Future Trends and Innovations in Catalyst Development
Future trends in catalyst development emphasize the design of pyrophoric catalysts with enhanced safety features to enable their use in broader industrial applications, including energy storage and green hydrogen production. Innovations include the integration of advanced nanostructuring techniques and computational modeling to tailor catalyst surface properties for improved reactivity and stability under ambient conditions. Non-pyrophoric catalysts are being optimized for robustness and selectivity through bio-inspired approaches and hybrid materials, aiming to reduce environmental impact and increase lifecycle efficiency.
Pyrophoric catalyst vs non-pyrophoric catalyst Infographic
 libmatt.com
libmatt.com