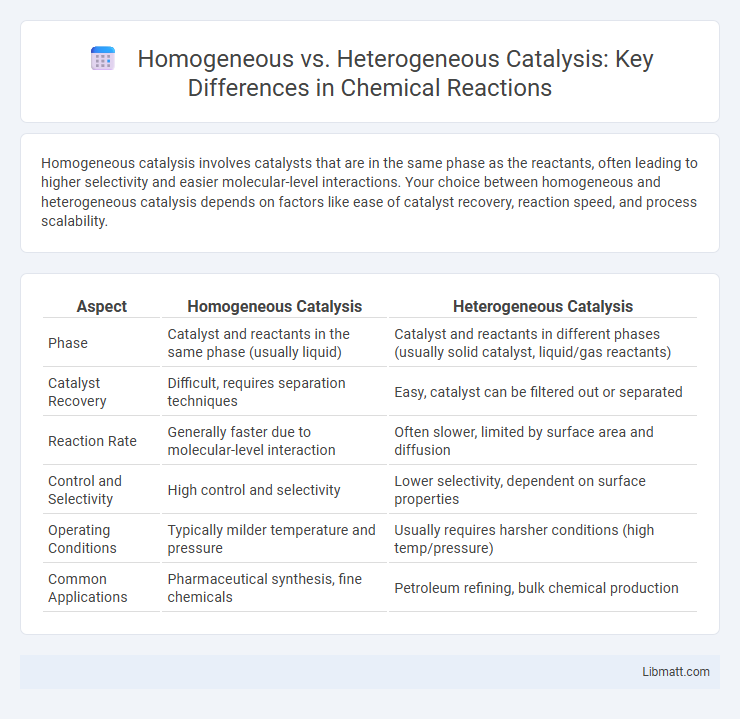
Homogeneous catalysis involves catalysts that are in the same phase as the reactants, often leading to higher selectivity and easier molecular-level interactions. Your choice between homogeneous and heterogeneous catalysis depends on factors like ease of catalyst recovery, reaction speed, and process scalability.
Table of Comparison
| Aspect | Homogeneous Catalysis | Heterogeneous Catalysis |
|---|---|---|
| Phase | Catalyst and reactants in the same phase (usually liquid) | Catalyst and reactants in different phases (usually solid catalyst, liquid/gas reactants) |
| Catalyst Recovery | Difficult, requires separation techniques | Easy, catalyst can be filtered out or separated |
| Reaction Rate | Generally faster due to molecular-level interaction | Often slower, limited by surface area and diffusion |
| Control and Selectivity | High control and selectivity | Lower selectivity, dependent on surface properties |
| Operating Conditions | Typically milder temperature and pressure | Usually requires harsher conditions (high temp/pressure) |
| Common Applications | Pharmaceutical synthesis, fine chemicals | Petroleum refining, bulk chemical production |
Introduction to Catalysis
Catalysis accelerates chemical reactions by lowering activation energy, enhancing reaction rates without being consumed. Homogeneous catalysis involves catalysts in the same phase as reactants, often liquid, enabling uniform interaction and high selectivity. Heterogeneous catalysis features catalysts in a different phase, typically solids interacting with gaseous or liquid reactants, facilitating easy separation and reuse.
Definition of Homogeneous Catalysis
Homogeneous catalysis involves catalysts that exist in the same phase as the reactants, typically in a liquid solution, facilitating molecular interactions at the molecular level. This catalytic process enhances reaction rates through uniform distribution and easy interaction between catalyst and substrates. Your choice of homogeneous catalysis is ideal for reactions requiring precise control and selectivity due to its single-phase nature and molecular-level efficiency.
Definition of Heterogeneous Catalysis
Heterogeneous catalysis involves catalysts in a different phase than the reactants, commonly solid catalysts interacting with liquid or gas reactants. This phase difference facilitates easy separation and reuse of the catalyst, enhancing industrial process efficiency. Your understanding of catalytic processes benefits from recognizing that heterogeneous catalysts provide surface sites where reactions preferentially occur, promoting selectivity and reaction rates.
Key Mechanistic Differences
Homogeneous catalysis involves catalysts and reactants in the same phase, typically liquid, allowing for molecular-level interactions and uniform active sites, which enhance selectivity and facilitate detailed mechanistic studies. Heterogeneous catalysis operates with catalysts in a different phase, often solid catalysts interacting with gas or liquid reactants, relying on adsorption and surface reactions involving complex active sites and mass transfer limitations. The key mechanistic difference lies in the nature of catalyst-reactant contact: homogeneous catalysis exhibits homogeneous molecular interactions, while heterogeneous catalysis involves surface phenomena governed by adsorption, desorption, and surface diffusion processes.
Common Examples of Homogeneous Catalysts
Common examples of homogeneous catalysts include transition metal complexes such as Wilkinson's catalyst (chlorotris(triphenylphosphine)rhodium(I)) used in hydrogenation reactions and Grubbs' catalysts employed in olefin metathesis. Enzymes represent biological homogeneous catalysts, accelerating biochemical reactions with high specificity. Homogeneous catalysis typically involves catalysts dissolving in the same phase as reactants, often facilitating easier mechanistic study and fine-tuning of catalytic activity.
Common Examples of Heterogeneous Catalysts
Common examples of heterogeneous catalysts include platinum and palladium used in catalytic converters, iron in Haber-Bosch ammonia synthesis, and vanadium oxide in sulfuric acid production. These catalysts facilitate reactions by providing active surfaces for molecules to adsorb and react, improving efficiency and selectivity in industrial processes. Your choice of catalyst type impacts reaction conditions and product purity, with heterogeneous catalysis offering easy separation from reaction mixtures.
Advantages of Homogeneous Catalysis
Homogeneous catalysis offers superior selectivity and efficiency due to the catalyst and reactants sharing the same phase, usually liquid, which facilitates better molecular interaction. The ability to fine-tune catalyst structures at the molecular level allows for enhanced control over reaction pathways and product outcomes. Your processes benefit from easier catalyst modification and often milder reaction conditions compared to heterogeneous catalysis.
Advantages of Heterogeneous Catalysis
Heterogeneous catalysis offers significant advantages such as easy separation of catalysts from reaction mixtures, enabling catalyst reuse and reducing production costs. It provides enhanced thermal stability and facilitates continuous processes in industrial applications due to its solid catalyst form. The method also allows for precise control over catalyst surface properties, improving selectivity and reaction rates in large-scale manufacturing.
Challenges and Limitations of Each Method
Homogeneous catalysis often faces challenges related to catalyst recovery and separation due to its solubility in the reaction medium, which complicates recycling and increases operational costs. Heterogeneous catalysis, while easier to separate, is limited by lower selectivity and potential catalyst deactivation caused by fouling or sintering on solid surfaces. Both methods encounter stability issues under harsh reaction conditions, impacting catalyst lifespan and overall process efficiency.
Industrial Applications and Future Trends
Homogeneous catalysis is extensively used in fine chemical production and pharmaceutical synthesis due to its high selectivity and ease of reaction control, while heterogeneous catalysis dominates large-scale industrial processes such as petroleum refining and automotive exhaust treatment because of its robustness and catalyst recyclability. Emerging trends include the development of hybrid catalytic systems combining the advantages of both types and the integration of artificial intelligence for catalyst design, enhancing efficiency and sustainability in your industrial applications. Future advancements are expected to focus on greener catalysts, improved catalyst lifespan, and scalable processes that reduce environmental impact and operational costs.
Homogeneous catalysis vs heterogeneous catalysis Infographic
 libmatt.com
libmatt.com