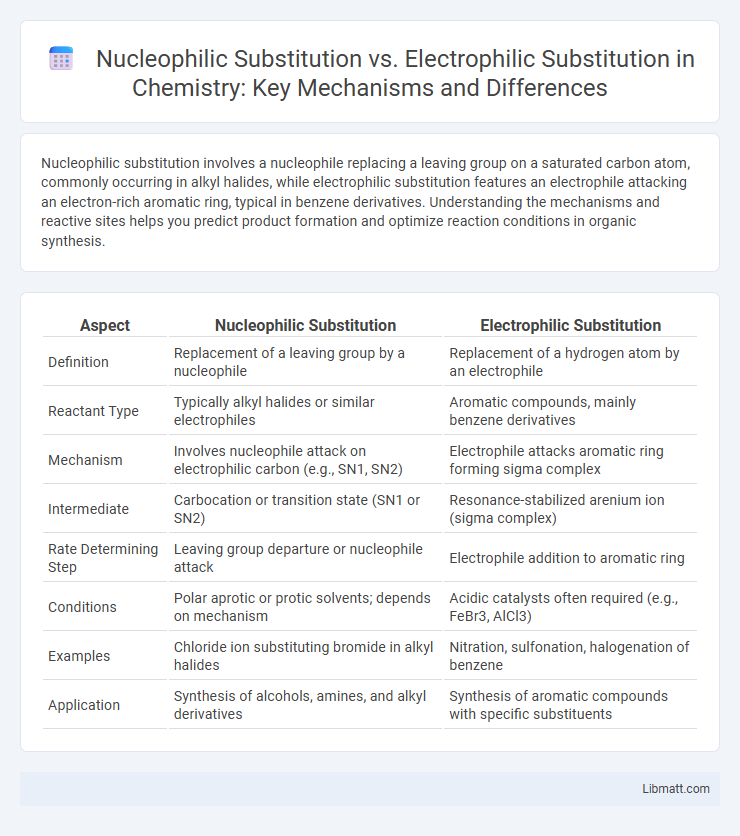
Nucleophilic substitution involves a nucleophile replacing a leaving group on a saturated carbon atom, commonly occurring in alkyl halides, while electrophilic substitution features an electrophile attacking an electron-rich aromatic ring, typical in benzene derivatives. Understanding the mechanisms and reactive sites helps you predict product formation and optimize reaction conditions in organic synthesis.
Table of Comparison
| Aspect | Nucleophilic Substitution | Electrophilic Substitution |
|---|---|---|
| Definition | Replacement of a leaving group by a nucleophile | Replacement of a hydrogen atom by an electrophile |
| Reactant Type | Typically alkyl halides or similar electrophiles | Aromatic compounds, mainly benzene derivatives |
| Mechanism | Involves nucleophile attack on electrophilic carbon (e.g., SN1, SN2) | Electrophile attacks aromatic ring forming sigma complex |
| Intermediate | Carbocation or transition state (SN1 or SN2) | Resonance-stabilized arenium ion (sigma complex) |
| Rate Determining Step | Leaving group departure or nucleophile attack | Electrophile addition to aromatic ring |
| Conditions | Polar aprotic or protic solvents; depends on mechanism | Acidic catalysts often required (e.g., FeBr3, AlCl3) |
| Examples | Chloride ion substituting bromide in alkyl halides | Nitration, sulfonation, halogenation of benzene |
| Application | Synthesis of alcohols, amines, and alkyl derivatives | Synthesis of aromatic compounds with specific substituents |
Introduction to Substitution Reactions
Substitution reactions involve the replacement of an atom or group in a molecule with another atom or group, categorized as nucleophilic substitution and electrophilic substitution based on the attacking species. Nucleophilic substitution occurs when a nucleophile donates an electron pair to an electron-deficient carbon, commonly seen in alkyl halides. Electrophilic substitution typically involves an electrophile attacking an electron-rich aromatic ring, such as in the halogenation or nitration of benzene.
Understanding Nucleophilic Substitution
Nucleophilic substitution involves a nucleophile attacking an electron-deficient carbon atom, replacing a leaving group, which is fundamental in organic reactions like SN1 and SN2 mechanisms. Understanding nucleophilic substitution helps you predict reaction outcomes, as factors such as substrate structure, nucleophile strength, and solvent type critically influence reaction rates. Mastering these concepts enhances your ability to manipulate molecular transformations in synthesis and pharmaceutical development.
Mechanism of Nucleophilic Substitution (SN1 & SN2)
Nucleophilic substitution involves the replacement of a leaving group by a nucleophile through two primary mechanisms: SN1 and SN2. The SN1 mechanism proceeds via a two-step process involving the formation of a carbocation intermediate, leading to racemization in chiral centers and is favored in tertiary alkyl halides and polar protic solvents. In contrast, SN2 is a single-step, bimolecular reaction where the nucleophile attacks the electrophilic carbon from the opposite side of the leaving group, causing inversion of configuration and is typical in primary alkyl halides and polar aprotic solvents.
Key Features of Electrophilic Substitution
Electrophilic substitution features the attack of an electrophile on an electron-rich aromatic ring, typically involving benzene or its derivatives. The process retains the aromaticity of the ring by temporarily forming a positively charged sigma complex before the substitution completes. Your understanding of this mechanism is crucial for mastering reactions like nitration, halogenation, and sulfonation in aromatic chemistry.
Mechanism of Electrophilic Substitution (SEAr & SEAl)
The mechanism of electrophilic aromatic substitution (SEAr) involves the initial attack of an electrophile on the aromatic ring, forming a sigma complex (arenium ion) followed by deprotonation to restore aromaticity. Electrophilic aliphatic substitution (SEAl) proceeds via the formation of a carbocation intermediate after the electrophile displaces a leaving group on an aliphatic substrate. Both mechanisms are characterized by the electrophile targeting electron-rich sites but differ in intermediate stability and the nature of the substrate involved.
Factors Influencing Nucleophilic vs Electrophilic Substitution
Factors influencing nucleophilic substitution include the nature of the nucleophile, substrate structure, and solvent polarity, with stronger nucleophiles and polar aprotic solvents enhancing reaction rates. Electrophilic substitution is primarily governed by the electrophile's strength, substrate electron density, and the presence of activating or deactivating groups on aromatic rings. Temperature and reaction conditions also modulate the selectivity between nucleophilic and electrophilic pathways.
Common Examples and Applications
Nucleophilic substitution reactions are commonly exemplified by the SN1 and SN2 mechanisms, frequently utilized in synthesizing pharmaceuticals and agrochemicals, such as the preparation of alkyl halides and amines. Electrophilic substitution typically occurs in aromatic compounds, with nitration, halogenation, and sulfonation serving as classic examples vital for manufacturing dyes, explosives, and polymers. These reactions enable precise molecular modifications, making them essential in organic synthesis and industrial chemistry applications.
Comparing Reaction Conditions and Substrates
Nucleophilic substitution reactions typically occur with substrates bearing good leaving groups, such as alkyl halides, under polar aprotic or protic solvents that stabilize the nucleophile and leaving group. Electrophilic substitution primarily involves aromatic compounds with electron-rich p-systems, requiring an electrophile often generated in situ under acidic conditions to facilitate substitution. Your choice of reaction depends on the substrate structure and the specific reaction conditions that favor nucleophile or electrophile attack.
Importance in Organic Synthesis
Nucleophilic substitution reactions are crucial in organic synthesis for introducing functional groups by replacing leaving groups with nucleophiles, enabling the formation of alcohols, amines, and halides. Electrophilic substitution is key in modifying aromatic compounds, allowing selective addition of electrophiles to aromatic rings, which is vital for synthesizing dyes, pharmaceuticals, and polymers. Both mechanisms expand molecular complexity and diversify synthetic pathways, facilitating targeted synthesis in pharmaceuticals, agrochemicals, and materials science.
Summary: Nucleophilic vs Electrophilic Substitution
Nucleophilic substitution involves a nucleophile replacing a leaving group in a molecule, commonly occurring in alkyl halides and characterized by the attack on an electron-deficient carbon. Electrophilic substitution typically occurs in aromatic compounds, where an electrophile replaces a hydrogen atom on the aromatic ring, preserving the ring's stability. Understanding the distinct mechanisms helps you predict reaction outcomes and choose appropriate reagents in synthesis.
nucleophilic substitution vs electrophilic substitution Infographic
 libmatt.com
libmatt.com