Urea fertilizer provides a concentrated source of nitrogen that rapidly converts to ammonium, promoting efficient nutrient uptake and strong plant growth. Nitrate fertilizer supplies nitrogen in an immediately available form, supporting quick assimilation and enhanced photosynthesis, which can boost crop yield and quality.
Table of Comparison
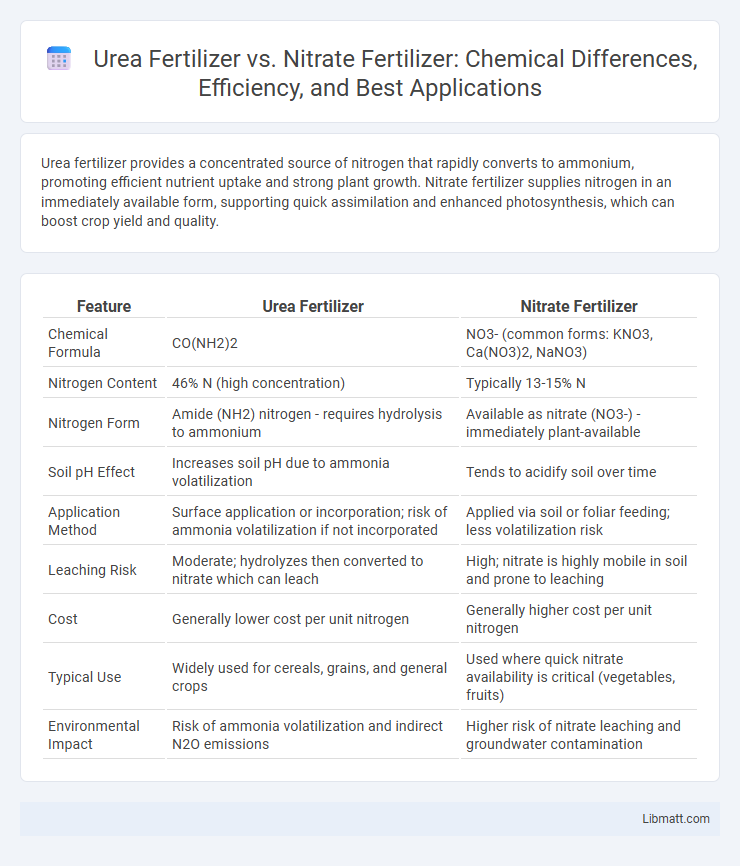
| Feature | Urea Fertilizer | Nitrate Fertilizer |
|---|---|---|
| Chemical Formula | CO(NH2)2 | NO3- (common forms: KNO3, Ca(NO3)2, NaNO3) |
| Nitrogen Content | 46% N (high concentration) | Typically 13-15% N |
| Nitrogen Form | Amide (NH2) nitrogen - requires hydrolysis to ammonium | Available as nitrate (NO3-) - immediately plant-available |
| Soil pH Effect | Increases soil pH due to ammonia volatilization | Tends to acidify soil over time |
| Application Method | Surface application or incorporation; risk of ammonia volatilization if not incorporated | Applied via soil or foliar feeding; less volatilization risk |
| Leaching Risk | Moderate; hydrolyzes then converted to nitrate which can leach | High; nitrate is highly mobile in soil and prone to leaching |
| Cost | Generally lower cost per unit nitrogen | Generally higher cost per unit nitrogen |
| Typical Use | Widely used for cereals, grains, and general crops | Used where quick nitrate availability is critical (vegetables, fruits) |
| Environmental Impact | Risk of ammonia volatilization and indirect N2O emissions | Higher risk of nitrate leaching and groundwater contamination |
Introduction to Urea and Nitrate Fertilizers
Urea fertilizer contains 46% nitrogen in a stable, concentrated form that promotes rapid nitrogen uptake by plants, making it one of the most widely used nitrogen sources worldwide. Nitrate fertilizers, such as calcium nitrate and potassium nitrate, supply nitrogen in the nitrate ion form (NO3-) which is immediately available for plant absorption and less prone to volatilization compared to urea. Your choice between urea and nitrate fertilizers depends on soil conditions, crop type, and environmental factors to optimize nitrogen use efficiency and crop yield.
Chemical Composition and Structure
Urea fertilizer contains 46% nitrogen in the form of a stable amide group (CO(NH2)2), providing a high nitrogen content with low volatilization risk. Nitrate fertilizers primarily consist of nitrogen in the nitrate ion (NO3-), which is highly soluble and immediately available for plant uptake but more prone to leaching. The chemical structure of urea promotes gradual nitrogen release through microbial hydrolysis, whereas nitrate fertilizers supply nitrogen in a directly usable ionic form.
Mechanisms of Nitrogen Release
Urea fertilizer releases nitrogen primarily through hydrolysis, where the enzyme urease converts urea into ammonium, subsequently available for plant uptake or further nitrification. Nitrate fertilizer provides nitrogen directly in the nitrate form, allowing immediate absorption by plants without the need for enzymatic conversion. The differing mechanisms impact nitrogen availability timing, influencing fertilizer efficiency and environmental nitrogen losses.
Soil Interaction and Nitrogen Availability
Urea fertilizer undergoes rapid hydrolysis in the soil, converting to ammonium and subsequently to nitrate, making nitrogen available more gradually compared to nitrate fertilizers that provide immediate nitrate ions for plant uptake. Soil pH and microbial activity significantly influence urea's transformation, often leading to temporary ammonia volatilization losses, whereas nitrate fertilizers are more prone to leaching in sandy soils due to their anionic nature. Your choice between urea and nitrate fertilizers should consider soil texture, moisture, and environmental conditions to optimize nitrogen availability and minimize losses.
Effect on Crop Yield and Growth
Urea fertilizer, containing 46% nitrogen, offers a concentrated nitrogen source that promotes rapid vegetative growth and higher crop yields when properly applied. Nitrate fertilizers, such as calcium nitrate, provide readily available nitrogen in nitrate form, enhancing root development and supporting steady growth, particularly beneficial in alkaline soils. Studies indicate urea's nitrogen must be converted to nitrate in soil before uptake, causing a slight delay in nutrient availability compared to nitrate fertilizers, which can result in varied effects on crop yield depending on soil type and environmental conditions.
Environmental Impact and Leaching Potential
Urea fertilizer releases ammonia and can volatilize into the atmosphere, contributing to greenhouse gas emissions, while also posing a risk of nitrate leaching into groundwater if improperly managed. Nitrate fertilizers are highly soluble and mobile in soil, increasing the potential for nitrate leaching, which can contaminate water sources and cause eutrophication. Urea's nitrogen is converted to nitrate in the soil through microbial activity, making its environmental impact dependent on application timing and soil conditions, whereas nitrate fertilizers have an immediate leaching risk.
Cost Analysis and Economic Considerations
Urea fertilizer generally offers a lower cost per unit of nitrogen compared to nitrate fertilizers, making it a more economical choice for large-scale agricultural applications. However, nitrate fertilizers can provide faster nitrogen availability, potentially improving crop yields and offsetting their higher initial cost in certain cropping systems. Your decision should balance upfront costs with crop response and soil conditions to maximize return on investment.
Application Methods and Best Practices
Urea fertilizer is commonly applied through broadcasting, banding, or foliar spraying, with incorporation into the soil shortly after application to minimize nitrogen loss through volatilization. Nitrate fertilizers, such as calcium nitrate, are best applied via fertigation, side-dressing, or drip irrigation, allowing for precise nutrient delivery and reducing leaching risks. To optimize your crop yield, choose the application method based on soil type, crop stage, and environmental conditions to ensure maximum nitrogen efficiency and minimize environmental impact.
Suitability for Different Soil Types
Urea fertilizer is highly suitable for well-drained, sandy, and loamy soils due to its high nitrogen content and rapid conversion to ammonium, which supports quick nutrient uptake in these soil types. Nitrate fertilizers perform better in heavier clay soils and alkaline conditions where nitrate ions remain more available and less prone to volatilization or leaching. Soil pH and moisture retention capacity significantly influence the efficiency of both fertilizers, with nitrate fertilizers favored in soils prone to rapid urea hydrolysis and nitrogen losses.
Summary: Choosing the Right Nitrogen Fertilizer
Urea fertilizer contains 46% nitrogen in a highly concentrated form, making it cost-effective and efficient for rapid plant uptake, while nitrate fertilizers provide nitrogen in a readily available form that reduces volatilization losses. The choice between urea and nitrate fertilizers depends on soil pH, crop type, irrigation practices, and environmental conditions, with urea favored in well-managed systems and nitrate preferred in sandy soils prone to leaching. Understanding these factors ensures optimal nitrogen availability, enhances crop yield, and minimizes environmental impact.
Urea fertilizer vs nitrate fertilizer Infographic
 libmatt.com
libmatt.com