Oxidation involves the loss of electrons and an increase in oxidation state, while reduction entails the gain of electrons and a decrease in oxidation state. Understanding these complementary processes is essential for mastering redox reactions and their applications in chemistry.
Table of Comparison
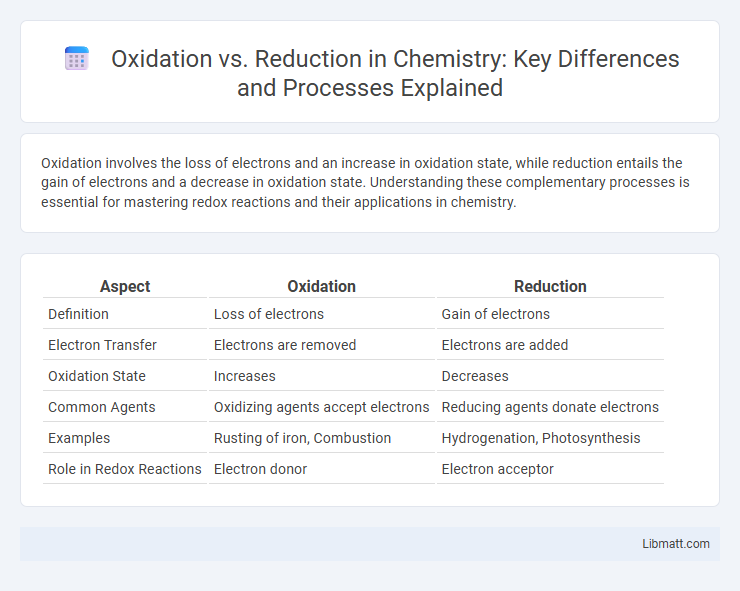
| Aspect | Oxidation | Reduction |
|---|---|---|
| Definition | Loss of electrons | Gain of electrons |
| Electron Transfer | Electrons are removed | Electrons are added |
| Oxidation State | Increases | Decreases |
| Common Agents | Oxidizing agents accept electrons | Reducing agents donate electrons |
| Examples | Rusting of iron, Combustion | Hydrogenation, Photosynthesis |
| Role in Redox Reactions | Electron donor | Electron acceptor |
Introduction to Oxidation and Reduction
Oxidation and reduction are fundamental chemical reactions involving electron transfer between substances. Oxidation refers to the loss of electrons, while reduction involves the gain of electrons, often occurring simultaneously in redox reactions. Understanding these processes is essential for applications in energy production, corrosion prevention, and metabolic pathways, directly impacting your knowledge of chemical and biological systems.
Defining Oxidation: Key Concepts
Oxidation involves the loss of electrons by a molecule, atom, or ion, increasing its oxidation state and often associated with the addition of oxygen or removal of hydrogen. Key concepts include electron transfer processes, formation of oxides, and energy changes in redox reactions. Understanding oxidation is essential for analyzing chemical reactions in fields like electrochemistry, biochemistry, and industrial applications.
Understanding Reduction: Essential Principles
Reduction involves the gain of electrons by a molecule, atom, or ion, leading to a decrease in its oxidation state. This essential process plays a critical role in redox reactions, where it typically occurs alongside oxidation, the loss of electrons. Understanding reduction is fundamental in fields such as chemistry, biology, and industrial applications like metal extraction and energy storage.
Historical Perspective: The Evolution of Redox Concepts
The evolution of redox concepts began in the 18th century with Antoine Lavoisier's identification of oxygen's role in combustion and rusting, establishing oxidation as the gain of oxygen. Later, in the 19th century, scientists like Humphry Davy expanded the understanding to include electron transfer, defining reduction as the gain of electrons and oxidation as their loss. The development of electrochemistry by Michael Faraday further refined the redox framework, linking chemical reactions to electrical energy and enabling quantitative analysis of redox processes.
Electron Transfer: The Core Mechanism
Oxidation involves the loss of electrons, while reduction is characterized by the gain of electrons during a chemical reaction. This electron transfer is the core mechanism driving redox reactions, essential in processes like cellular respiration and corrosion. Understanding how your substances exchange electrons enables better control of energy flow and chemical transformations.
Redox Reactions in Everyday Life
Redox reactions play a crucial role in everyday life through processes like cellular respiration, where glucose is oxidized to produce energy, and photosynthesis, which involves the reduction of carbon dioxide to form glucose. Corrosion of metals, such as rusting of iron, is another common example of oxidation, while antioxidants in food help protect cells by preventing excessive oxidation. These redox reactions underpin vital biological functions and influence many industrial applications, emphasizing their importance in daily environmental and physiological phenomena.
Common Examples of Oxidation and Reduction
Rusting of iron exemplifies oxidation where iron reacts with oxygen to form iron oxide, while the bleaching process illustrates reduction by removing or altering color through the gain of electrons. Cellular respiration involves oxidation as glucose is broken down releasing energy, whereas photosynthesis demonstrates reduction by converting carbon dioxide into glucose using sunlight energy. Common industrial applications include the oxidation of ethanol to acetic acid and the reduction of metal ores to pure metals during extraction.
Industrial Applications of Redox Processes
Redox processes play a crucial role in industrial applications such as metal extraction, wastewater treatment, and chemical manufacturing. Oxidation reactions are commonly used to remove impurities in metal refining, while reduction is essential in producing pure metals from their ores. Your understanding of these processes can optimize efficiency and sustainability in industrial operations.
Oxidation vs. Reduction in Biological Systems
Oxidation in biological systems involves the loss of electrons, often resulting in the breakdown of molecules to release energy, while reduction entails the gain of electrons, enabling the synthesis of essential biomolecules. Cellular respiration exemplifies this by oxidizing glucose to produce ATP, with oxygen acting as the final electron acceptor in the electron transport chain. Understanding the balance between oxidation and reduction is crucial for maintaining your body's metabolic functions and preventing oxidative stress-related damage.
Summary: Key Differences and Takeaways
Oxidation involves the loss of electrons, increasing an element's oxidation state, while reduction entails the gain of electrons, decreasing it. Key differences include oxidation producing electrons and reduction consuming them, with oxidation often releasing energy and reduction requiring energy input. Your understanding of these complementary processes is crucial for grasping redox reactions in chemistry and their applications in biological and industrial contexts.
oxidation vs reduction Infographic
 libmatt.com
libmatt.com