Aseptic processing involves assembling and packaging sterile products in a controlled environment to prevent contamination, ensuring product sterility without exposing it to high heat. Your choice between aseptic processing and terminal sterilization depends on product sensitivity, as terminal sterilization uses intense heat or chemicals applied after packaging to eliminate microorganisms but may damage heat-sensitive materials.
Table of Comparison
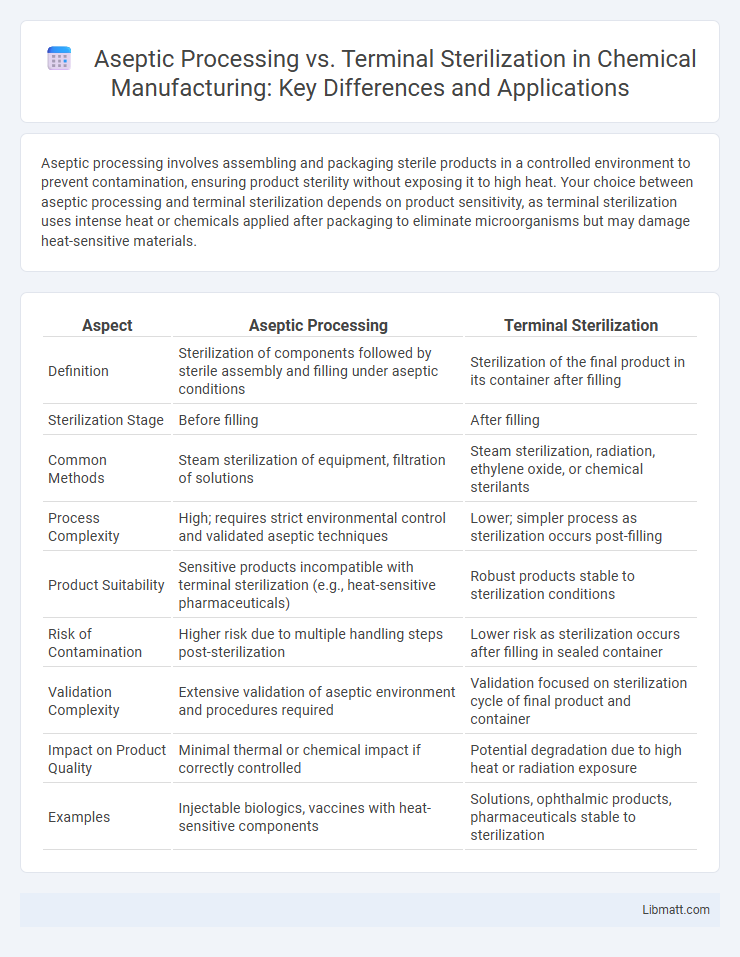
| Aspect | Aseptic Processing | Terminal Sterilization |
|---|---|---|
| Definition | Sterilization of components followed by sterile assembly and filling under aseptic conditions | Sterilization of the final product in its container after filling |
| Sterilization Stage | Before filling | After filling |
| Common Methods | Steam sterilization of equipment, filtration of solutions | Steam sterilization, radiation, ethylene oxide, or chemical sterilants |
| Process Complexity | High; requires strict environmental control and validated aseptic techniques | Lower; simpler process as sterilization occurs post-filling |
| Product Suitability | Sensitive products incompatible with terminal sterilization (e.g., heat-sensitive pharmaceuticals) | Robust products stable to sterilization conditions |
| Risk of Contamination | Higher risk due to multiple handling steps post-sterilization | Lower risk as sterilization occurs after filling in sealed container |
| Validation Complexity | Extensive validation of aseptic environment and procedures required | Validation focused on sterilization cycle of final product and container |
| Impact on Product Quality | Minimal thermal or chemical impact if correctly controlled | Potential degradation due to high heat or radiation exposure |
| Examples | Injectable biologics, vaccines with heat-sensitive components | Solutions, ophthalmic products, pharmaceuticals stable to sterilization |
Introduction to Sterile Manufacturing Methods
Aseptic processing and terminal sterilization are critical sterile manufacturing methods ensuring product safety and efficacy. Aseptic processing involves sterilizing individual components separately and assembling them in a sterile environment to prevent contamination during manufacturing. Terminal sterilization uses sterilizing agents or processes, such as autoclaving or radiation, directly on the final sealed product to achieve sterility.
Defining Aseptic Processing
Aseptic processing involves the sterilization of individual components such as equipment, containers, and product ingredients separately, followed by their assembly and filling in a sterile environment to prevent contamination. This method is essential for heat-sensitive pharmaceuticals and biologics that cannot withstand terminal sterilization, which exposes the final product to high temperatures to achieve sterility. Understanding your product's tolerance to heat and the risk of contamination is crucial when choosing between aseptic processing and terminal sterilization for optimal product safety.
Understanding Terminal Sterilization
Terminal sterilization involves subjecting a product to a final sterilization step, typically using methods such as steam, ethylene oxide, or gamma irradiation, to achieve a Sterility Assurance Level (SAL) of 10^-6. This process is preferred when drug formulations and packaging materials can withstand sterilization conditions without degradation. Understanding terminal sterilization is crucial for ensuring product safety, regulatory compliance, and maintaining the stability and efficacy of pharmaceuticals.
Key Differences Between Aseptic Processing and Terminal Sterilization
Aseptic processing involves sterilizing individual components separately before assembly in a sterile environment, while terminal sterilization sterilizes the final product in its sealed container. Key differences include the risk of contamination, with aseptic processing requiring stringent environmental controls to maintain sterility, whereas terminal sterilization offers a more robust sterilization method but may not be suitable for heat-sensitive products. Your choice between these methods depends on product formulation, packaging compatibility, and regulatory requirements for sterility assurance.
Common Applications in Pharmaceutical Manufacturing
Aseptic processing is commonly used for heat-sensitive pharmaceutical products such as vaccines, biologics, and injectable solutions that cannot withstand terminal sterilization temperatures. Terminal sterilization, involving high-temperature steam or radiation, is preferred for heat-stable products like solid oral dosage forms and certain parenteral solutions to ensure sterility assurance levels (SAL) of 10^-6. Pharmaceutical manufacturers select aseptic processing or terminal sterilization based on product stability, regulatory requirements, and the risk of microbial contamination during packaging.
Regulatory Requirements and Standards
Regulatory requirements for aseptic processing and terminal sterilization are defined by agencies such as the FDA, EMA, and USP, emphasizing strict compliance with current Good Manufacturing Practices (cGMP). Aseptic processing mandates rigorous environmental monitoring, sterilization validation, and personnel training to prevent contamination during product filling, as outlined in USP <1116> and FDA 21 CFR Part 211. Terminal sterilization follows validated sterilization cycles, often using methods like steam or radiation, ensuring final product sterility with validated parameters per ISO 11135 and 11137 standards.
Risks and Contamination Control
Aseptic processing involves assembling sterile components in a controlled environment, posing higher risks of contamination due to exposure during processing stages, necessitating stringent environmental monitoring and operator controls. Terminal sterilization applies a validated sterilization method to the final product in its sealed container, significantly reducing contamination risks by eliminating microorganisms after packaging. Contamination control in aseptic processing relies heavily on cleanroom technology, airflow management, and surface sanitization, whereas terminal sterilization primarily depends on sterilant efficacy and packaging integrity.
Advantages and Limitations of Each Method
Aseptic processing enables the sterilization of heat-sensitive pharmaceuticals by separately sterilizing product components and assembling them in a sterile environment, ensuring high product quality and flexibility for complex formulations, yet it demands rigorous environmental controls and skilled operators, increasing operational complexity and cost. Terminal sterilization offers a robust, reliable sterilization by treating the final product in its sealed container, reducing contamination risks and simplifying validation; however, it may cause degradation of heat-labile products and is unsuitable for some sensitive drug formulations. Selection between these methods depends on product stability, regulatory requirements, and manufacturing capabilities to balance efficacy, safety, and economic feasibility.
Quality Assurance and Process Validation
Aseptic processing requires rigorous environmental monitoring and strict control of sterile conditions to ensure product sterility, demanding comprehensive process validation including media fills that simulate production runs. Terminal sterilization offers the advantage of a validated kill step that can compensate for minor process deviations, providing a higher assurance of sterility and reducing reliance on aseptic handling controls. Quality assurance in terminal sterilization emphasizes validation of sterilization cycles and packaging integrity, whereas aseptic processing quality assurance focuses on preventing contamination during sterile component assembly and filling.
Choosing the Right Sterilization Approach for Your Product
Selecting between aseptic processing and terminal sterilization depends on your product's heat sensitivity and packaging compatibility, as aseptic processing suits heat-sensitive pharmaceuticals by sterilizing components separately before filling, while terminal sterilization involves sterilizing the final sealed product. Terminal sterilization offers superior sterility assurance but may degrade certain drug formulations, making aseptic processing ideal for biologics and complex therapies. Assessing product stability, container integrity, and regulatory requirements ensures you choose the optimal sterilization method that maintains efficacy and safety.
Aseptic processing vs terminal sterilization Infographic
 libmatt.com
libmatt.com