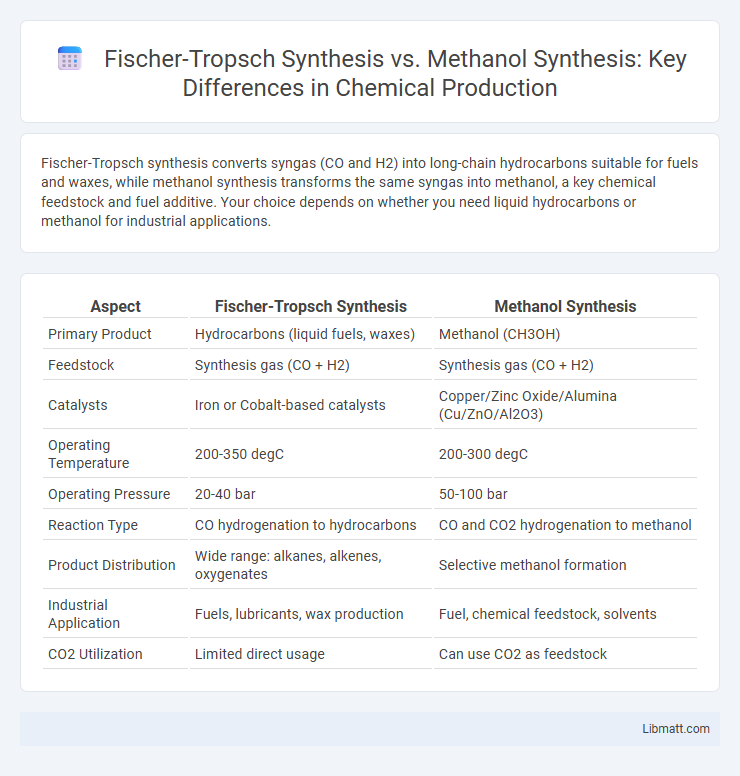
Fischer-Tropsch synthesis converts syngas (CO and H2) into long-chain hydrocarbons suitable for fuels and waxes, while methanol synthesis transforms the same syngas into methanol, a key chemical feedstock and fuel additive. Your choice depends on whether you need liquid hydrocarbons or methanol for industrial applications.
Table of Comparison
| Aspect | Fischer-Tropsch Synthesis | Methanol Synthesis |
|---|---|---|
| Primary Product | Hydrocarbons (liquid fuels, waxes) | Methanol (CH3OH) |
| Feedstock | Synthesis gas (CO + H2) | Synthesis gas (CO + H2) |
| Catalysts | Iron or Cobalt-based catalysts | Copper/Zinc Oxide/Alumina (Cu/ZnO/Al2O3) |
| Operating Temperature | 200-350 degC | 200-300 degC |
| Operating Pressure | 20-40 bar | 50-100 bar |
| Reaction Type | CO hydrogenation to hydrocarbons | CO and CO2 hydrogenation to methanol |
| Product Distribution | Wide range: alkanes, alkenes, oxygenates | Selective methanol formation |
| Industrial Application | Fuels, lubricants, wax production | Fuel, chemical feedstock, solvents |
| CO2 Utilization | Limited direct usage | Can use CO2 as feedstock |
Introduction to Fischer–Tropsch and Methanol Synthesis
Fischer-Tropsch synthesis is a catalytic chemical process that converts syngas, a mixture of carbon monoxide and hydrogen, into hydrocarbons, primarily used for producing liquid fuels and waxes. Methanol synthesis involves the catalytic conversion of syngas into methanol, a key feedstock for chemicals and alternative fuels. Both processes operate under high pressure and temperature conditions, utilizing metal catalysts such as cobalt or iron for Fischer-Tropsch and copper-based catalysts for methanol synthesis, with significant industrial applications in gas-to-liquid and gas-to-chemicals technologies.
Historical Background and Industrial Significance
Fischer-Tropsch synthesis, developed in the 1920s by Franz Fischer and Hans Tropsch, was initially driven by Germany's need for synthetic fuels during World War II, establishing its historical significance in converting coal, natural gas, or biomass into liquid hydrocarbons. Methanol synthesis emerged earlier, in the early 20th century, and became industrially significant as a versatile chemical feedstock and renewable fuel, with large-scale production evolving through catalytic innovations. Your understanding of these processes highlights their roles in energy security and sustainable fuel production, where Fischer-Tropsch focuses on hydrocarbon fuels and methanol synthesis on a key building block for chemicals and energy carriers.
Core Chemical Reactions and Mechanisms
Fischer-Tropsch synthesis converts syngas (CO and H2) into hydrocarbons through surface-catalyzed polymerization involving metal catalysts like iron or cobalt, emphasizing chain growth via CO dissociation and hydrogenation. Methanol synthesis also utilizes syngas but primarily involves CO and CO2 hydrogenation on copper-based catalysts, forming methanol through intermediate formate and formaldehyde species. Understanding these distinct reaction pathways helps optimize your choice of catalyst and operating conditions for fuel versus chemical feedstock production.
Feedstock Requirements and Versatility
Fischer-Tropsch synthesis primarily requires syngas, a mixture of carbon monoxide and hydrogen, typically derived from coal, natural gas, or biomass, offering flexibility in feedstock sources. Methanol synthesis also uses syngas as feedstock but demands a more precise H2 to CO ratio for efficient conversion, often favoring natural gas reforming. Fischer-Tropsch's ability to process diverse feedstocks makes it more versatile for producing long-chain hydrocarbons, while methanol synthesis is optimized for producing methanol with high selectivity from cleaner, more controlled syngas inputs.
Catalyst Types and Performance Comparison
Fischer-Tropsch synthesis employs iron or cobalt catalysts to convert syngas into long-chain hydrocarbons, excelling in producing diesel and waxes with high selectivity and stability under high-pressure conditions. Methanol synthesis primarily uses copper-based catalysts supported on zinc oxide and alumina, offering efficient conversion of syngas to methanol with high activity at lower temperatures and pressures. Your choice depends on the desired fuel or chemical output, catalyst lifespan, and operational costs, with Fischer-Tropsch favoring hydrocarbon fuels and methanol synthesis optimized for liquid chemical intermediates.
Process Conditions: Temperature, Pressure, and Efficiency
Fischer-Tropsch synthesis operates at temperatures between 150-300degC and pressures of 20-40 bar, favoring longer-chain hydrocarbons with moderate efficiency in converting syngas to liquid fuels. Methanol synthesis typically occurs at lower temperatures of 200-270degC and higher pressures of 50-100 bar, achieving higher efficiency for converting syngas primarily into methanol due to optimized catalyst activity. Your choice between these processes depends on desired product output and energy efficiency under specific operational conditions.
Product Range and Applications
Fischer-Tropsch synthesis produces a broad spectrum of hydrocarbons, including liquid fuels like diesel, gasoline, and waxes, primarily used in transportation and industrial applications. Methanol synthesis generates a single key product, methanol, which serves as a versatile feedstock for chemicals, fuel additives, and energy carriers. Your choice depends on whether you need diverse hydrocarbon fuels or a specialized chemical intermediate for further processing.
Environmental Impact and Sustainability
Fischer-Tropsch synthesis produces cleaner fuels like synthetic diesel and waxes with lower sulfur and aromatic content, reducing hazardous emissions compared to conventional fossil fuels. Methanol synthesis enables versatile energy storage and can be produced sustainably from CO2 and renewable hydrogen, supporting carbon recycling and lower greenhouse gas emissions. Your choice between these processes influences the carbon footprint and aligns with long-term sustainability goals in fuel and chemical production.
Economic Considerations and Scalability
Fischer-Tropsch synthesis offers flexibility in feedstock utilization and generates a broad range of hydrocarbons, contributing to higher capital costs and complex scale-up challenges compared to methanol synthesis, which benefits from established catalyst systems and lower operating expenses. Methanol synthesis features higher volumetric productivity and simpler reactor designs, making it economically advantageous for large-scale production with lower capital investment. The scalability of Fischer-Tropsch plants often demands significant infrastructure and integration with upstream gasification, whereas methanol plants can be more modular and rapidly deployed, enhancing their economic feasibility in diverse market conditions.
Future Prospects and Technological Innovations
Fischer-Tropsch synthesis advances through integration with renewable hydrogen sources, boosting carbon-neutral fuel production and leveraging modular reactor designs for scalability. Methanol synthesis technology rapidly evolves by incorporating advanced catalysts and electrochemical methods to enhance efficiency and reduce carbon footprint in chemical manufacturing. Both processes benefit from digitalization and AI-driven process optimization, promising enhanced operational control and resource utilization in future industrial applications.
Fischer–Tropsch synthesis vs methanol synthesis Infographic
 libmatt.com
libmatt.com