Exothermic reactions release heat energy to the surroundings, causing the temperature to rise, while endothermic reactions absorb heat, resulting in a temperature decrease. Your understanding of these processes helps in predicting how energy flows during chemical changes and their practical applications.
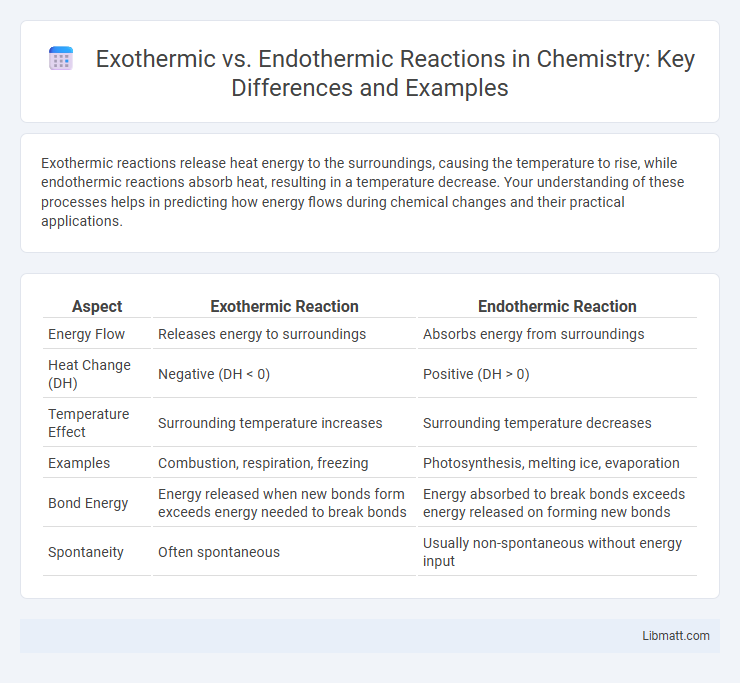
Table of Comparison
| Aspect | Exothermic Reaction | Endothermic Reaction |
|---|---|---|
| Energy Flow | Releases energy to surroundings | Absorbs energy from surroundings |
| Heat Change (DH) | Negative (DH < 0) | Positive (DH > 0) |
| Temperature Effect | Surrounding temperature increases | Surrounding temperature decreases |
| Examples | Combustion, respiration, freezing | Photosynthesis, melting ice, evaporation |
| Bond Energy | Energy released when new bonds form exceeds energy needed to break bonds | Energy absorbed to break bonds exceeds energy released on forming new bonds |
| Spontaneity | Often spontaneous | Usually non-spontaneous without energy input |
Introduction to Chemical Reactions
Exothermic reactions release energy, typically in the form of heat, as chemical bonds form, resulting in a temperature increase in the surroundings. Endothermic reactions absorb energy, requiring heat intake to break chemical bonds, causing a temperature decrease in the environment. These fundamental processes illustrate the energy flow during chemical reactions, crucial for understanding reaction spontaneity and thermodynamics.
Defining Exothermic and Endothermic Reactions
Exothermic reactions release energy, usually in the form of heat, resulting in a temperature increase in the surroundings, such as combustion or respiration. Endothermic reactions absorb energy from the surroundings, causing a temperature drop, exemplified by processes like photosynthesis and melting ice. Understanding these reactions helps you predict energy changes and reaction behavior in chemical and physical processes.
Key Differences Between Exothermic and Endothermic Reactions
Exothermic reactions release energy to the surroundings, usually in the form of heat or light, causing an increase in the temperature of the environment, while endothermic reactions absorb energy, resulting in a temperature decrease. Exothermic processes include combustion and condensation, whereas endothermic processes involve melting, evaporation, and photosynthesis. The enthalpy change (DH) is negative for exothermic reactions and positive for endothermic reactions, reflecting the energy flow direction.
Energy Changes in Chemical Reactions
Exothermic reactions release energy into the surroundings, typically in the form of heat, resulting in a temperature increase as bonds form and excess energy is expelled. Endothermic reactions absorb energy from the environment, causing a temperature decrease as energy is required to break bonds within the reactants. Understanding these energy changes in chemical reactions helps you predict reaction behavior and manage thermal effects in various applications.
Examples of Exothermic Reactions
Combustion of fuels like gasoline and natural gas are classic examples of exothermic reactions, releasing significant heat energy. The respiration process in living organisms, where glucose reacts with oxygen to produce carbon dioxide, water, and energy, also exemplifies exothermic reactions. Freezing of water into ice is another exothermic process, as it releases latent heat to the surroundings.
Examples of Endothermic Reactions
Photosynthesis, where plants absorb sunlight to convert carbon dioxide and water into glucose, is a prime example of an endothermic reaction. Other common examples include the melting of ice and the evaporation of water, which require heat absorption to change states. Understanding these processes helps you grasp how energy intake drives chemical and physical transformations.
Everyday Applications of Exothermic Reactions
Exothermic reactions release heat, making them essential in everyday applications such as hand warmers, combustion engines, and food digestion. Your body relies on exothermic processes to generate energy and maintain warmth, while industries use these reactions for welding and heating purposes. Understanding exothermic reactions enhances the practical use of energy-efficient technologies in daily life.
Everyday Applications of Endothermic Reactions
Endothermic reactions absorb heat from their surroundings, making them essential in everyday applications such as refrigeration and cold packs, where they provide cooling by drawing in thermal energy. Photosynthesis in plants is a natural endothermic process converting solar energy into chemical energy, supporting ecosystems and agriculture. You can observe endothermic reactions in cooking methods like boiling and melting, where heat absorption changes food states and textures.
Factors Influencing Reaction Type
Temperature significantly influences whether a reaction is exothermic or endothermic, with higher temperatures favoring endothermic processes due to increased energy absorption. The nature of reactants, including bond energies and molecular structure, determines if energy is released or required during reaction progress. Pressure and catalysts also impact reaction pathways, often altering the activation energy and thereby influencing the thermodynamic direction of the reaction.
Summary: Choosing Exothermic vs Endothermic Reactions
Exothermic reactions release heat, making them ideal for processes where energy output is desired, such as combustion and respiration. Endothermic reactions absorb heat, requiring continuous energy input, suitable for applications like photosynthesis and refrigeration. Your choice between exothermic and endothermic reactions depends on whether you need to generate heat or absorb it to drive the process efficiently.
Exothermic reaction vs endothermic reaction Infographic
 libmatt.com
libmatt.com