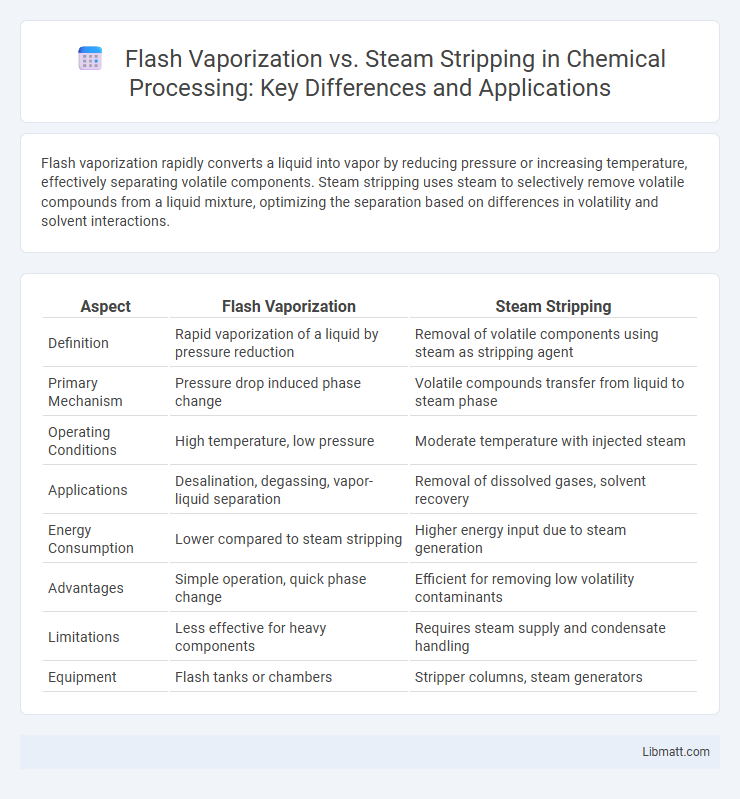
Flash vaporization rapidly converts a liquid into vapor by reducing pressure or increasing temperature, effectively separating volatile components. Steam stripping uses steam to selectively remove volatile compounds from a liquid mixture, optimizing the separation based on differences in volatility and solvent interactions.
Table of Comparison
| Aspect | Flash Vaporization | Steam Stripping |
|---|---|---|
| Definition | Rapid vaporization of a liquid by pressure reduction | Removal of volatile components using steam as stripping agent |
| Primary Mechanism | Pressure drop induced phase change | Volatile compounds transfer from liquid to steam phase |
| Operating Conditions | High temperature, low pressure | Moderate temperature with injected steam |
| Applications | Desalination, degassing, vapor-liquid separation | Removal of dissolved gases, solvent recovery |
| Energy Consumption | Lower compared to steam stripping | Higher energy input due to steam generation |
| Advantages | Simple operation, quick phase change | Efficient for removing low volatility contaminants |
| Limitations | Less effective for heavy components | Requires steam supply and condensate handling |
| Equipment | Flash tanks or chambers | Stripper columns, steam generators |
Introduction to Flash Vaporization and Steam Stripping
Flash vaporization rapidly converts a liquid mixture into vapor by sudden pressure reduction, enabling separation based on volatility differences. Steam stripping involves injecting steam to volatize and remove volatile compounds from a liquid, enhancing contaminant removal efficiency. Both processes are widely used in chemical engineering for phase separation and purification tasks.
Fundamental Principles of Flash Vaporization
Flash vaporization relies on rapidly reducing the pressure of a heated liquid, causing a portion of it to instantly vaporize due to sudden thermodynamic disequilibrium. This process separates volatile components by exploiting differences in boiling points without added heat, making it energy-efficient. Your understanding of flash vaporization is crucial for optimizing separation processes in chemical engineering and industrial applications.
Key Concepts in Steam Stripping
Steam stripping utilizes steam to separate volatile compounds from liquids by reducing their partial pressure, enhancing mass transfer efficiency in the stripping column. Unlike flash vaporization, which rapidly vaporizes a liquid at reduced pressure or increased temperature, steam stripping relies on continuous steam flow to selectively remove impurities or solvents. Your process efficiency in steam stripping depends on factors like steam temperature, flow rate, and the volatility of target compounds.
Process Flow: Flash Vaporization Explained
Flash vaporization involves rapidly reducing the pressure of a liquid feed, causing immediate partial vaporization and separation of volatile components from the liquid phase. In contrast, steam stripping uses continuous steam injection to volatilize impurities, facilitating their removal through a countercurrent flow in a stripping column. Your choice between these processes depends on factors like feed composition, desired purity, and equipment constraints.
Process Flow: How Steam Stripping Works
Steam stripping involves injecting steam into a liquid mixture to selectively volatilize and remove volatile compounds, enhancing separation efficiency. The process flow begins with preheating the feed, followed by contacting it with rising steam in a stripping column where volatile components vaporize and ascend while non-volatile components remain liquid and flow downward. The vaporized compounds are then condensed and collected, achieving effective purification through continuous countercurrent mass transfer.
Equipment and Design Considerations
Flash vaporization equipment typically involves a flash drum or flash tank designed to rapidly reduce pressure and separate vapor from liquid phases, requiring precise control of temperature and pressure to optimize phase separation. Steam stripping systems utilize packed or tray columns that demand careful design to ensure efficient contact between steam and the liquid mixture, promoting the selective removal of volatile components. Material selection and corrosion resistance are critical design factors for both processes due to exposure to high temperatures and potentially aggressive chemical environments.
Efficiency and Performance Comparison
Flash vaporization offers rapid phase separation with moderate energy consumption but is less effective in removing volatile components compared to steam stripping. Steam stripping provides higher efficiency in contaminant removal due to continuous steam contact, enhancing mass transfer rates and achieving lower residual concentrations. Performance evaluations indicate steam stripping is more suitable for complex mixtures requiring thorough purification, while flash vaporization is advantageous for quick, energy-efficient separation of simpler systems.
Operational Applications in Industry
Flash vaporization is widely used in petrochemical refineries for separating light hydrocarbon fractions by rapidly reducing pressure, enabling efficient phase change and component separation. Steam stripping is commonly applied in wastewater treatment and chemical manufacturing to remove volatile organic compounds by injecting steam, enhancing mass transfer and contaminant removal. Both processes optimize separation efficiency but are selected based on feed composition, desired purity, and operational cost in industrial applications.
Advantages and Limitations of Each Method
Flash vaporization offers rapid phase separation by instantly vaporizing a portion of a liquid mixture under reduced pressure, which is energy-efficient and suitable for feed streams with high volatility. Limitations include its inability to achieve complete component separation and dependence on specific vapor-liquid equilibrium characteristics. Steam stripping provides effective removal of volatile contaminants through direct steam contact, enabling thorough purification, but requires higher energy input and careful steam management to avoid product degradation.
Selecting the Right Method: Flash Vaporization vs Steam Stripping
Selecting the right method between flash vaporization and steam stripping depends on the specific characteristics of your mixture and separation goals. Flash vaporization is ideal for systems where rapid pressure reduction causes volatile components to vaporize instantly, leveraging temperature and composition differences. Steam stripping suits scenarios requiring targeted removal of volatile contaminants using direct steam injection, providing efficient mass transfer in packed or tray columns.
flash vaporization vs steam stripping Infographic
 libmatt.com
libmatt.com