Alkylation involves the transfer of an alkyl group to a molecule, typically increasing hydrocarbon chains and enhancing hydrophobicity, while acylation introduces an acyl group, often modifying molecular polarity and reactivity by adding carbonyl functionality. Understanding the differences between these reactions helps optimize synthetic pathways and tailor compounds for specific chemical properties.
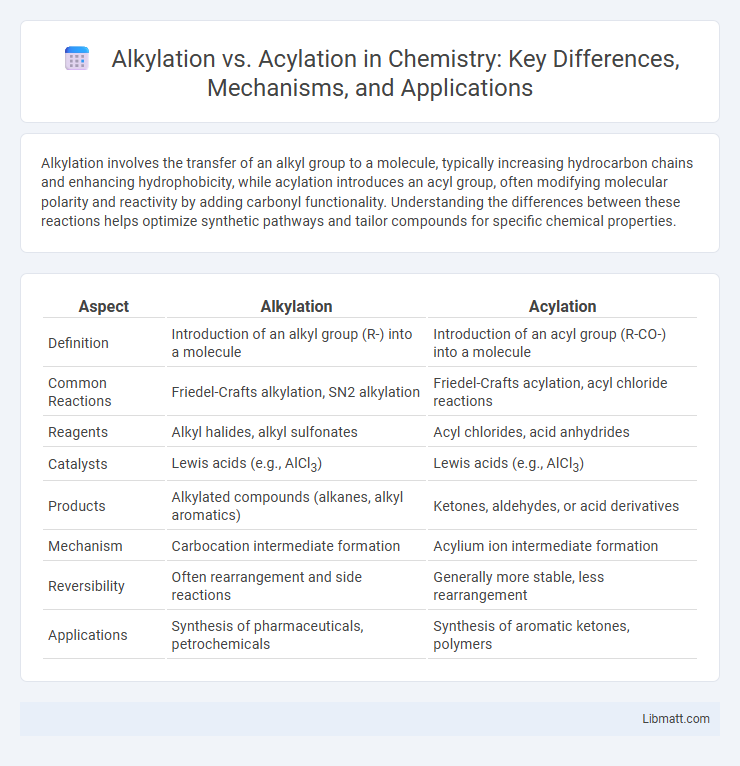
Table of Comparison
| Aspect | Alkylation | Acylation |
|---|---|---|
| Definition | Introduction of an alkyl group (R-) into a molecule | Introduction of an acyl group (R-CO-) into a molecule |
| Common Reactions | Friedel-Crafts alkylation, SN2 alkylation | Friedel-Crafts acylation, acyl chloride reactions |
| Reagents | Alkyl halides, alkyl sulfonates | Acyl chlorides, acid anhydrides |
| Catalysts | Lewis acids (e.g., AlCl3) | Lewis acids (e.g., AlCl3) |
| Products | Alkylated compounds (alkanes, alkyl aromatics) | Ketones, aldehydes, or acid derivatives |
| Mechanism | Carbocation intermediate formation | Acylium ion intermediate formation |
| Reversibility | Often rearrangement and side reactions | Generally more stable, less rearrangement |
| Applications | Synthesis of pharmaceuticals, petrochemicals | Synthesis of aromatic ketones, polymers |
Introduction to Alkylation and Acylation
Alkylation and acylation are fundamental organic chemistry reactions that introduce alkyl or acyl groups into molecules, respectively. Alkylation involves the addition of alkyl groups, typically using alkyl halides or alkenes, to form new carbon-carbon bonds, enhancing molecular complexity. Acylation introduces acyl groups derived from acid chlorides or anhydrides, often modifying physical and chemical properties by forming ketones, aldehydes, or esters in your target compounds.
Fundamental Concepts: What Are Alkylation and Acylation?
Alkylation is a chemical reaction where an alkyl group is transferred to a molecule, typically enhancing hydrocarbon chain length and modifying chemical properties. Acylation involves the introduction of an acyl group into a compound, often resulting in the formation of ketones, aldehydes, or esters with altered reactivity. Both processes are fundamental in organic synthesis, enabling the structural modification of molecules for applications in pharmaceuticals, polymers, and petrochemicals.
Key Differences Between Alkylation and Acylation
Alkylation involves the transfer of an alkyl group to a substrate, typically enhancing the hydrocarbon chain length, while acylation introduces an acyl group, often forming carbonyl-containing functional groups like ketones or esters. Your reaction conditions, catalysts such as Lewis acids for alkylation, and reagents like acid chlorides or anhydrides for acylation differ significantly, influencing the type of bond formation and product stability. The key differences lie in the electrophiles used, the product functionalities, and their applications in organic synthesis and industrial chemistry.
Mechanisms of Alkylation Reactions
Alkylation mechanisms typically involve the formation of a carbocation intermediate or the direct nucleophilic attack on an alkyl halide, where electrophilic alkyl groups are transferred to aromatic rings or other nucleophiles. In Friedel-Crafts alkylation, the Lewis acid catalyst such as AlCl3 facilitates carbocation formation from alkyl halides, enabling electrophilic aromatic substitution. Carbocation stability and rearrangements significantly influence the product distribution and regioselectivity in alkylation reactions.
Mechanisms of Acylation Reactions
Acylation reactions typically proceed via electrophilic substitution, where an acyl group is introduced into an aromatic ring or substrate through the formation of an acylium ion (RCO+), facilitated by Lewis acids like AlCl3. This mechanism involves the generation of a resonance-stabilized electrophile, which attacks the nucleophilic site on the substrate, followed by deprotonation to restore aromaticity. Understanding this pathway enables you to control reaction conditions for efficient synthesis of ketones or amides in organic chemistry.
Common Reagents Used in Alkylation and Acylation
Alkylation commonly utilizes reagents such as alkyl halides (e.g., methyl chloride, ethyl bromide) and Lewis acids like aluminum chloride (AlCl3) to facilitate the transfer of alkyl groups to aromatic compounds. Acylation employs acyl chlorides (e.g., acetyl chloride, benzoyl chloride) or acid anhydrides with Lewis acids such as AlCl3 to introduce acyl groups, forming ketones or amides. Both reactions are key electrophilic aromatic substitution processes, with reagent selection critical for controlling reactivity and product specificity.
Industrial Applications of Alkylation vs Acylation
Alkylation processes are crucial in the petrochemical industry for producing high-octane gasoline components and synthetic lubricants, enhancing fuel efficiency and engine performance. Acylation is widely used in pharmaceutical and polymer industries to synthesize ketones, aromatic aldehydes, and intermediates for drug manufacturing, enabling the production of various medicinal and polymeric materials. Both reactions are fundamental in organic synthesis but serve distinct industrial purposes based on their chemical modification of hydrocarbons.
Advantages and Limitations of Each Method
Alkylation offers the advantage of introducing alkyl groups into a molecule, enhancing hydrophobicity and improving stability, but it often requires strong acid catalysts and may lead to carbocation rearrangements, limiting selectivity. Acylation provides precise control over functional group addition, increasing molecule polarity and reactivity, though it frequently involves harsher conditions and can result in lower yields due to side reactions like hydrolysis. Understanding the specific advantages and limitations of alkylation and acylation helps you choose the appropriate method to optimize your synthetic pathway efficiently.
Environmental and Safety Considerations
Alkylation processes often involve hazardous reagents such as alkyl halides, which pose risks of toxicity and flammability, requiring strict handling and proper ventilation to minimize environmental impact. Acylation typically uses acyl chlorides or anhydrides, generating corrosive byproducts like hydrogen chloride, necessitating effective neutralization and waste management to prevent environmental contamination. Implementing closed systems and adequate personal protective equipment reduces exposure risks for both alkylation and acylation, promoting safer industrial operations.
Conclusion: Choosing Between Alkylation and Acylation
Choosing between alkylation and acylation depends on the desired structural modification and reactivity of your target molecule. Alkylation introduces alkyl groups, enhancing hydrocarbon chains and increasing lipophilicity, while acylation adds acyl groups, often improving stability and introducing carbonyl functionality. Understanding these differences ensures optimal chemical synthesis tailored to your specific application needs.
Alkylation vs acylation Infographic
 libmatt.com
libmatt.com