Absorption involves a substance being fully taken up into the bulk of another material, while adsorption refers to molecules accumulating only on the surface of a material. Understanding the differences between absorption and adsorption is essential for optimizing processes in fields like chemistry, environmental science, and material engineering.
Table of Comparison
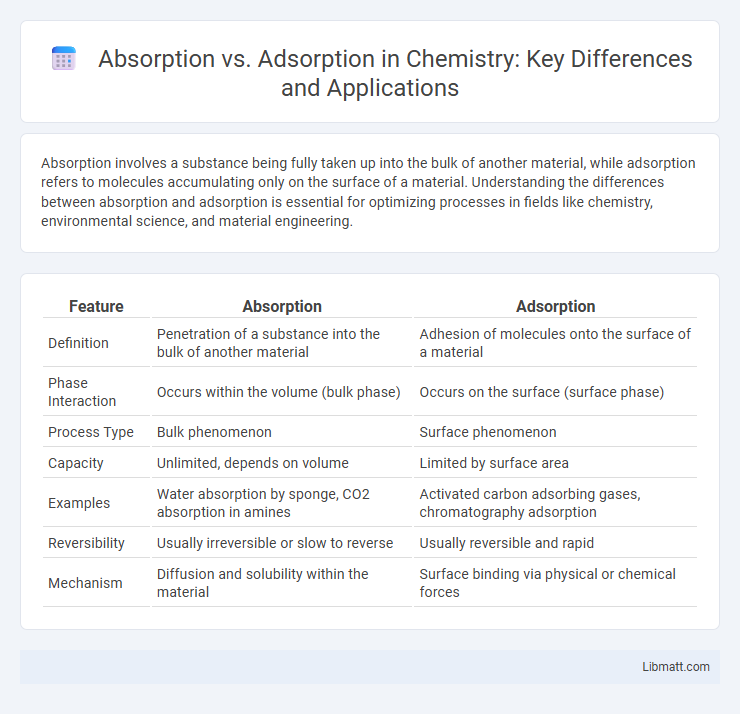
| Feature | Absorption | Adsorption |
|---|---|---|
| Definition | Penetration of a substance into the bulk of another material | Adhesion of molecules onto the surface of a material |
| Phase Interaction | Occurs within the volume (bulk phase) | Occurs on the surface (surface phase) |
| Process Type | Bulk phenomenon | Surface phenomenon |
| Capacity | Unlimited, depends on volume | Limited by surface area |
| Examples | Water absorption by sponge, CO2 absorption in amines | Activated carbon adsorbing gases, chromatography adsorption |
| Reversibility | Usually irreversible or slow to reverse | Usually reversible and rapid |
| Mechanism | Diffusion and solubility within the material | Surface binding via physical or chemical forces |
Introduction to Absorption and Adsorption
Absorption involves one substance being taken up into the volume or bulk of another, such as a liquid absorbing a gas. Adsorption occurs when molecules adhere only to the surface of a solid or liquid without penetrating its bulk. Understanding the difference between absorption and adsorption is essential for optimizing processes in industries like chemical engineering and environmental science, directly impacting your efficiency in applications like filtration or catalysis.
Definition of Absorption
Absorption is the process by which one substance is taken up into the interior of another substance, such as a gas or liquid dissolving into a solid or liquid. This phenomenon involves the entire volume of the absorbing material, allowing the absorbed molecules to penetrate deeply. You can observe absorption in everyday examples like a sponge soaking up water or carbon dioxide dissolving in blood.
Definition of Adsorption
Adsorption is the process by which molecules, atoms, or ions from a gas, liquid, or dissolved solid adhere only to the surface of a material, forming a thin film. This phenomenon differs from absorption, where substances penetrate into the bulk or interior of the absorbing material. Surface properties, such as porosity and surface area, significantly influence the efficiency and capacity of adsorption.
Key Differences Between Absorption and Adsorption
Absorption involves the penetration of a substance into the bulk phase of another material, while adsorption is the accumulation of molecules on the surface of a solid or liquid. Absorption affects the entire volume of the absorbing material, whereas adsorption is limited to the surface layer, making surface area and porosity critical factors. Your understanding of these key differences is essential in applications like catalysis, filtration, and chemical processing where material interaction plays a vital role.
Mechanisms of Absorption
Absorption involves the uptake of molecules by the bulk phase of a material, where the molecules penetrate into the volume rather than just adhering to the surface. This mechanism depends on factors such as solubility, diffusion rate, and material porosity, allowing substances to distribute uniformly throughout the absorbing medium. The efficiency of absorption is influenced by temperature, pressure, and the chemical affinity between the absorbate and absorbent.
Mechanisms of Adsorption
Adsorption involves the adhesion of atoms, ions, or molecules from a gas, liquid, or dissolved solid to a surface, primarily occurring at the interface due to physical or chemical interactions. Physical adsorption (physisorption) is driven by weak van der Waals forces and is usually reversible, while chemical adsorption (chemisorption) involves the formation of stronger chemical bonds, often resulting in an irreversible process. Factors influencing adsorption mechanisms include surface area, temperature, pressure, and the nature of adsorbent and adsorbate, critical for applications in catalysis, environmental purification, and material science.
Factors Influencing Absorption and Adsorption
Absorption is influenced by factors such as temperature, pressure, and the concentration gradient between phases, where higher temperatures typically enhance diffusion rates but may reduce solubility depending on the system. Adsorption depends on surface area, pore size, and the nature of the adsorbent and adsorbate, with increased surface area and optimal pore distribution significantly boosting adsorption capacity. Both processes are also affected by the chemical affinity between the materials involved, which determines the strength and extent of material uptake.
Real-World Applications of Absorption
Absorption plays a critical role in industries such as wastewater treatment, where pollutants are taken up by absorbent materials to purify water effectively. In chemical manufacturing, absorption enables the removal of gases like CO2 from emissions, improving environmental compliance. Understanding these real-world applications helps you optimize processes involving mass transfer for better efficiency and sustainability.
Practical Uses of Adsorption
Adsorption is widely used in water purification, where activated carbon filters remove impurities and contaminants by trapping them on their surface. Industrial gas processing relies on adsorption to separate and capture gases such as carbon dioxide or volatile organic compounds from air streams. In pharmaceuticals, adsorption plays a crucial role in controlled drug delivery systems by enabling drugs to adhere to carriers for gradual release.
Comparative Summary: Absorption vs Adsorption
Absorption involves the penetration of a substance into the bulk phase of a solid or liquid, whereas adsorption is the accumulation of molecules on the surface of a material. Your choice depends on whether you need interaction throughout the entire volume or only at the surface, with absorption offering bulk interaction and adsorption providing surface-specific effects. Key factors such as process reversibility, capacity, and application type distinguish absorption and adsorption in practical scenarios.
Absorption vs adsorption Infographic
 libmatt.com
libmatt.com