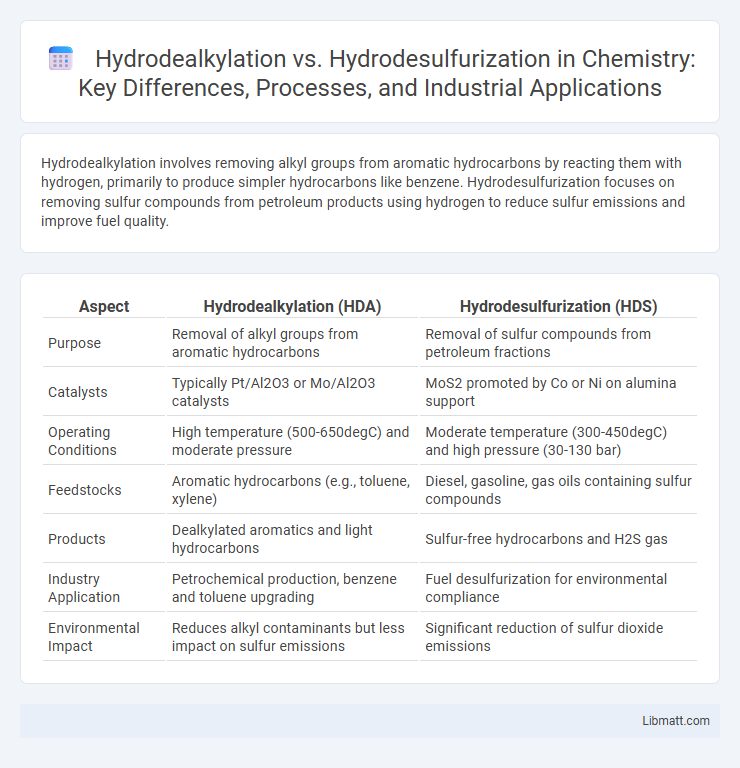
Hydrodealkylation involves removing alkyl groups from aromatic hydrocarbons by reacting them with hydrogen, primarily to produce simpler hydrocarbons like benzene. Hydrodesulfurization focuses on removing sulfur compounds from petroleum products using hydrogen to reduce sulfur emissions and improve fuel quality.
Table of Comparison
| Aspect | Hydrodealkylation (HDA) | Hydrodesulfurization (HDS) |
|---|---|---|
| Purpose | Removal of alkyl groups from aromatic hydrocarbons | Removal of sulfur compounds from petroleum fractions |
| Catalysts | Typically Pt/Al2O3 or Mo/Al2O3 catalysts | MoS2 promoted by Co or Ni on alumina support |
| Operating Conditions | High temperature (500-650degC) and moderate pressure | Moderate temperature (300-450degC) and high pressure (30-130 bar) |
| Feedstocks | Aromatic hydrocarbons (e.g., toluene, xylene) | Diesel, gasoline, gas oils containing sulfur compounds |
| Products | Dealkylated aromatics and light hydrocarbons | Sulfur-free hydrocarbons and H2S gas |
| Industry Application | Petrochemical production, benzene and toluene upgrading | Fuel desulfurization for environmental compliance |
| Environmental Impact | Reduces alkyl contaminants but less impact on sulfur emissions | Significant reduction of sulfur dioxide emissions |
Introduction to Hydrodealkylation and Hydrodesulfurization
Hydrodealkylation is a catalytic process that removes alkyl groups from aromatic compounds, primarily converting heavier hydrocarbons into lighter, more valuable products like benzene. Hydrodesulfurization is a catalytic chemical process used to remove sulfur from petroleum fractions to reduce sulfur dioxide emissions during fuel combustion. Both processes are essential in refining to improve fuel quality and meet environmental regulations by enhancing hydrocarbon purity and reducing harmful contaminants.
Process Overview: Hydrodealkylation Explained
Hydrodealkylation (HDA) is a catalytic process primarily used to convert alkylbenzenes into benzene by removing alkyl groups through hydrogenation at elevated temperatures and pressures. This method enhances the production of high-purity benzene for use in petrochemicals, distinguishing itself from hydrodesulfurization (HDS) which targets sulfur removal from hydrocarbons to reduce emissions. HDA relies on catalysts such as platinum or chromium-based materials to facilitate the cleavage of carbon-carbon bonds in alkyl side chains without significantly altering the sulfur content, optimizing feedstock for benzene production.
Process Overview: Hydrodesulfurization Explained
Hydrodesulfurization (HDS) is a catalytic chemical process used to remove sulfur compounds from petroleum products by reacting them with hydrogen gas over a solid catalyst, typically cobalt-molybdenum or nickel-molybdenum on alumina. This process significantly reduces sulfur emissions from fuels, meeting environmental regulations and improving fuel quality. While hydrodealkylation focuses on removing alkyl groups from aromatic hydrocarbons, hydrodesulfurization specifically targets sulfur-containing compounds like thiophenes to produce cleaner fuels and protect refining equipment.
Feedstock Requirements and Preparation
Hydrodealkylation requires feedstocks rich in alkylated aromatics, such as toluene or xylene, often necessitating pretreatment steps like catalytic reforming to enhance aromatic content. Hydrodesulfurization targets sulfur-containing compounds in various petroleum fractions, demanding careful feedstock filtration and stabilization to prevent catalyst poisoning. Your feedstock preparation must align with each process's specific chemical requirements to optimize reaction efficiency and catalyst lifespan.
Catalysts Used in Hydrodealkylation vs Hydrodesulfurization
Hydrodealkylation typically employs catalysts such as chromium-promoted alumina or platinum-based catalysts to facilitate the removal of alkyl groups from aromatic hydrocarbons. Hydrodesulfurization relies on molybdenum or tungsten sulfides promoted by cobalt or nickel supported on alumina, optimized for sulfur removal from petroleum fractions. The choice of catalysts directly influences the selectivity and efficiency in transforming hydrocarbons during refining processes.
Reaction Conditions and Operating Parameters
Hydrodealkylation (HDA) typically requires higher temperatures ranging from 500degC to 650degC and moderate hydrogen pressure between 20 to 50 bar, operating with catalysts such as chromium-promoted alumina to remove alkyl groups from aromatic compounds. Hydrodesulfurization (HDS) functions effectively at lower temperatures, generally between 300degC and 400degC, and higher hydrogen pressures around 30 to 130 bar, using catalysts like molybdenum disulfide promoted with cobalt or nickel to remove sulfur from hydrocarbon streams. Your choice between HDA and HDS depends on specific feedstock composition and desired product purity, as stringent operating parameters directly influence catalytic activity and contaminant removal efficiency.
Key Products and By-products Comparison
Hydrodealkylation primarily produces benzene by removing alkyl groups from alkylbenzenes, while hydrodesulfurization targets sulfur compounds to produce cleaner fuels with significantly reduced sulfur content. The key by-products of hydrodealkylation include light hydrocarbons such as methane and ethylene, whereas hydrodesulfurization mainly generates hydrogen sulfide gas as a by-product, requiring further treatment. Your choice between these processes depends on the desired product slate, with hydrodealkylation favoring aromatic hydrocarbons and hydrodesulfurization focusing on sulfur removal to meet environmental fuel standards.
Industrial Applications and End Uses
Hydrodealkylation (HDA) is primarily used in petrochemical industries to convert alkylbenzenes into benzene, a vital feedstock for producing styrene and other aromatic chemicals. Hydrodesulfurization (HDS) plays a crucial role in refining processes by removing sulfur compounds from fuels, thereby reducing sulfur emissions and meeting environmental regulations. Your choice between these processes depends on whether the goal is to enhance aromatic compound production or to produce cleaner fuels for transportation and industrial use.
Environmental Impact and Sulfur Removal Efficiency
Hydrodealkylation (HDA) primarily targets the removal of alkyl groups from aromatic hydrocarbons, producing cleaner fuels but with limited sulfur removal efficiency compared to hydrodesulfurization (HDS). Hydrodesulfurization is specifically designed for sulfur extraction, achieving sulfur content reduction to ultra-low levels, which substantially decreases sulfur dioxide emissions and acid rain formation. The environmental impact of HDS is generally more significant due to its superior sulfur removal capabilities, directly contributing to stricter compliance with sulfur regulations and reduced air pollution.
Economic Considerations and Process Selection
Hydrodealkylation (HDA) often demands higher operational costs compared to hydrodesulfurization (HDS) due to its more severe reaction conditions and catalyst requirements, influencing refinery economics significantly. Hydrodesulfurization is typically prioritized for its cost-effectiveness in sulfur removal, enhancing fuel quality while meeting environmental regulations at lower expense. Your process selection should weigh feedstock composition, product specifications, and economic impact, balancing HDA's complexity against HDS's established efficiency in sulfur removal.
Hydrodealkylation vs hydrodesulfurization Infographic
 libmatt.com
libmatt.com