The anode is the electrode where oxidation occurs, releasing electrons, while the cathode is the site of reduction, accepting electrons during electrochemical reactions. Understanding the roles of anode and cathode is essential for optimizing the performance of batteries, electrolytic cells, and other electrochemical systems.
Table of Comparison
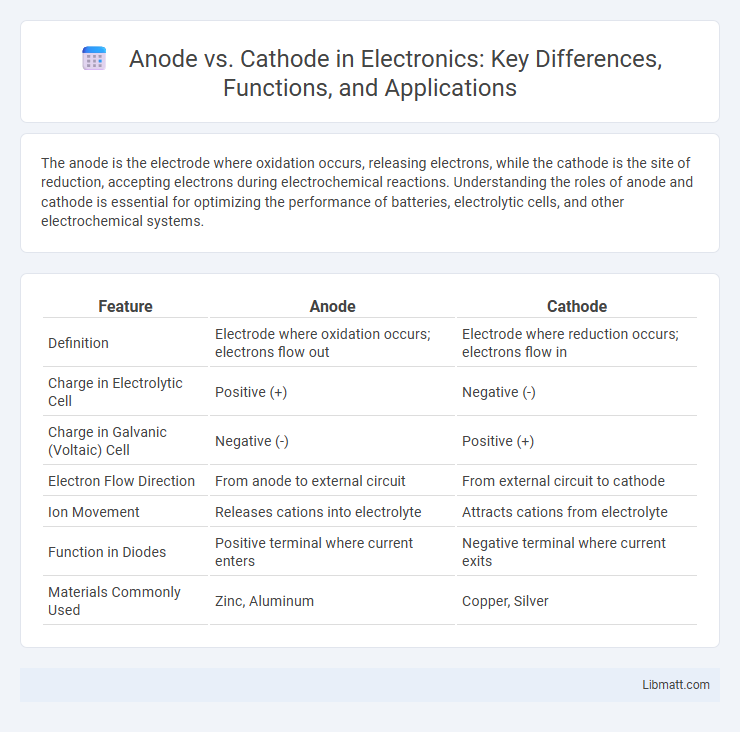
| Feature | Anode | Cathode |
|---|---|---|
| Definition | Electrode where oxidation occurs; electrons flow out | Electrode where reduction occurs; electrons flow in |
| Charge in Electrolytic Cell | Positive (+) | Negative (-) |
| Charge in Galvanic (Voltaic) Cell | Negative (-) | Positive (+) |
| Electron Flow Direction | From anode to external circuit | From external circuit to cathode |
| Ion Movement | Releases cations into electrolyte | Attracts cations from electrolyte |
| Function in Diodes | Positive terminal where current enters | Negative terminal where current exits |
| Materials Commonly Used | Zinc, Aluminum | Copper, Silver |
Introduction to Anode and Cathode
Anodes and cathodes are fundamental components in electrochemical cells, where the anode serves as the electrode that undergoes oxidation, releasing electrons, while the cathode is the electrode where reduction occurs, accepting electrons. In batteries, the anode typically acts as the negative terminal during discharge, whereas the cathode functions as the positive terminal, driving the flow of electric current through the external circuit. Understanding the distinct roles and electron flow at the anode and cathode is essential for optimizing battery design and electrochemical reactions.
Defining Anode and Cathode
The anode is the electrode where oxidation occurs, releasing electrons into the external circuit, while the cathode is the electrode where reduction takes place, accepting electrons from the circuit. In electrochemical cells, the anode is typically considered the negative terminal in galvanic cells and the positive terminal in electrolytic cells, whereas the cathode shows the opposite polarity. Understanding the roles of the anode and cathode is essential for optimizing your battery design and improving cell efficiency.
Fundamental Differences Between Anode and Cathode
Anodes are electrodes where oxidation occurs, releasing electrons into the external circuit, while cathodes are electrodes where reduction takes place, accepting electrons from the circuit. In galvanic cells, the anode is negative and the cathode is positive, whereas in electrolytic cells the anode is positive and the cathode is negative. The fundamental distinction lies in electron flow direction and the nature of electrochemical reactions: anodes emit electrons, and cathodes receive electrons.
Role of Anode and Cathode in Electrochemical Cells
The anode serves as the electrode where oxidation occurs, releasing electrons into the external circuit, while the cathode is the site of reduction, accepting electrons to complete the chemical reaction. In electrochemical cells, the anode typically functions as the negative terminal in galvanic cells and the positive terminal in electrolytic cells, influencing the direction of electron flow. Understanding the distinct roles of your anode and cathode is crucial for optimizing cell performance and efficiency in various applications.
Anode vs Cathode in Batteries
Anodes in batteries serve as the negative electrode during discharge, releasing electrons to the external circuit, while cathodes act as the positive electrode, accepting electrons and facilitating the reduction reaction. The choice of anode and cathode materials directly impacts battery capacity, voltage, and lifespan, with lithium-ion batteries commonly using graphite anodes and metal oxide cathodes. Understanding the anode vs cathode roles helps you evaluate battery performance and optimize energy storage solutions.
Applications in Electrolysis: Anode and Cathode Functions
In electrolysis, the anode serves as the positive electrode where oxidation occurs, releasing electrons and facilitating the formation of oxygen gas or other oxidation products. The cathode, acting as the negative electrode, attracts cations and drives reduction reactions, such as hydrogen gas production or metal deposition. Understanding the distinct roles of anode and cathode is crucial for optimizing your electrochemical processes and improving efficiency in applications like water splitting and metal plating.
Electron Flow: Direction and Significance
In electrochemical cells, electron flow occurs from the anode to the cathode, driven by the difference in electric potential. The anode serves as the negative electrode where oxidation releases electrons, while the cathode acts as the positive electrode where reduction consumes electrons. This directional electron flow is fundamental for generating electric current and sustaining redox reactions in batteries and electrolytic cells.
Real-world Examples of Anodes and Cathodes
In lithium-ion batteries, the anode typically consists of graphite, which stores lithium ions during charging, while the cathode is often made from lithium cobalt oxide or lithium iron phosphate, facilitating ion flow during discharge. In electroplating processes, the anode is a metal source such as copper or zinc that dissolves to coat a substrate at the cathode. Corrosion of iron involves the anode as the oxidizing metal surface where iron loses electrons, and the cathode as the site where the reduction reaction occurs, often involving oxygen and water.
Common Misconceptions about Anode and Cathode
Common misconceptions about anode and cathode often stem from confusing their roles in different devices; anode is incorrectly assumed to always be the positive electrode, while cathode is thought to be always negative. In reality, the anode is where oxidation occurs and electrons flow out of the device, making it positive in electrolytic cells but negative in galvanic cells, whereas the cathode is where reduction happens, attracting electrons and varying in charge depending on the cell type. Understanding these distinctions is crucial in electrochemistry to avoid errors in interpreting battery operation, electroplating, and corrosion processes.
Summary and Key Takeaways
Anode serves as the electrode where oxidation occurs, releasing electrons, while cathode is the electrode where reduction takes place, accepting electrons. In electrochemical cells, anode is typically the negative terminal in galvanic cells and positive in electrolytic cells, opposite for cathode. Understanding the roles of anode and cathode is crucial for designing batteries, electrolysis processes, and corrosion prevention strategies.
Anode vs Cathode Infographic
 libmatt.com
libmatt.com