Hydrogen embrittlement occurs when hydrogen atoms diffuse into a metal, causing it to become brittle and prone to sudden fracture under stress, while stress corrosion cracking involves the growth of cracks in a corrosive environment combined with tensile stress, leading to unexpected failure. Understanding the differences between these mechanisms is crucial for protecting your metal components in harsh environments and ensuring their long-term performance.
Table of Comparison
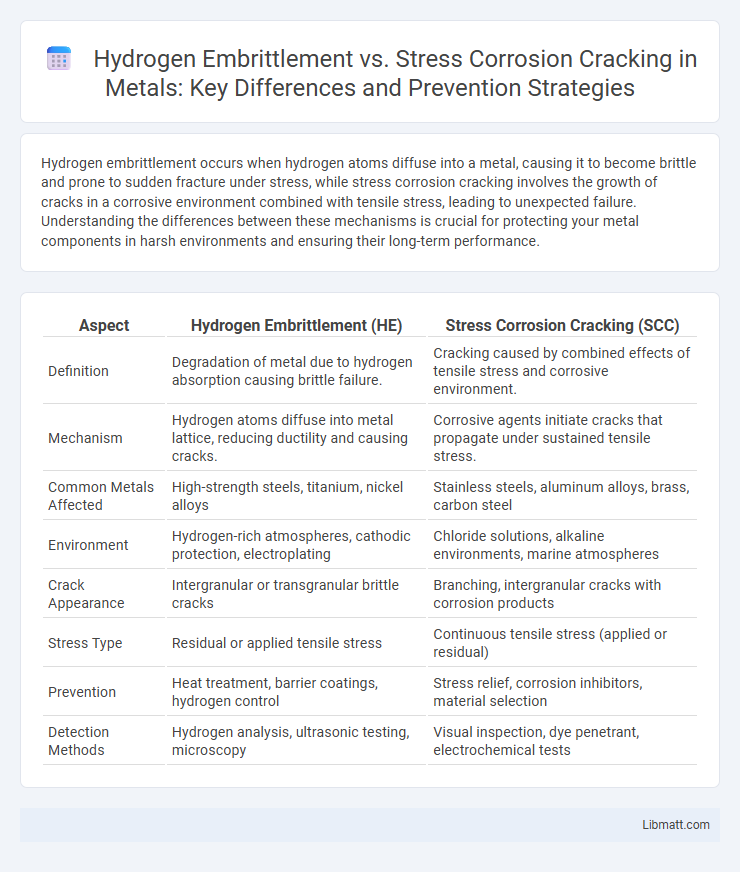
| Aspect | Hydrogen Embrittlement (HE) | Stress Corrosion Cracking (SCC) |
|---|---|---|
| Definition | Degradation of metal due to hydrogen absorption causing brittle failure. | Cracking caused by combined effects of tensile stress and corrosive environment. |
| Mechanism | Hydrogen atoms diffuse into metal lattice, reducing ductility and causing cracks. | Corrosive agents initiate cracks that propagate under sustained tensile stress. |
| Common Metals Affected | High-strength steels, titanium, nickel alloys | Stainless steels, aluminum alloys, brass, carbon steel |
| Environment | Hydrogen-rich atmospheres, cathodic protection, electroplating | Chloride solutions, alkaline environments, marine atmospheres |
| Crack Appearance | Intergranular or transgranular brittle cracks | Branching, intergranular cracks with corrosion products |
| Stress Type | Residual or applied tensile stress | Continuous tensile stress (applied or residual) |
| Prevention | Heat treatment, barrier coatings, hydrogen control | Stress relief, corrosion inhibitors, material selection |
| Detection Methods | Hydrogen analysis, ultrasonic testing, microscopy | Visual inspection, dye penetrant, electrochemical tests |
Introduction to Hydrogen Embrittlement and Stress Corrosion Cracking
Hydrogen embrittlement is a degradation process where metals, especially high-strength steels, become brittle and crack due to hydrogen atom infiltration. Stress corrosion cracking occurs when tensile stress and a corrosive environment combine to cause unexpected metal failure, often observed in stainless steels and aluminum alloys. Both mechanisms significantly reduce material durability and are critical concerns in aerospace, automotive, and infrastructure industries.
Defining Hydrogen Embrittlement: Mechanisms and Effects
Hydrogen embrittlement refers to a degradation process where metals become brittle and fracture due to the absorption and diffusion of hydrogen atoms. This phenomenon occurs through mechanisms like hydrogen-induced cracking, hydrogen-enhanced decohesion, and hydride formation, significantly reducing the material's ductility and tensile strength. The effects often manifest as premature failure in high-strength steels, impacting critical components in automotive, aerospace, and energy industries.
Overview of Stress Corrosion Cracking: Causes and Processes
Stress corrosion cracking (SCC) occurs when tensile stress and a corrosive environment combine to cause unexpected brittle failure in metals, often at low applied stresses. The process involves anodic dissolution or hydrogen embrittlement at the crack tip, leading to crack initiation and propagation along grain boundaries or through the metal matrix. Your understanding of SCC's causes and mechanisms is crucial for preventing catastrophic failures in pipelines, structural components, and industrial equipment exposed to aggressive environments.
Key Differences Between Hydrogen Embrittlement and Stress Corrosion Cracking
Hydrogen embrittlement predominantly occurs when hydrogen atoms diffuse into metals, causing brittleness and sudden failure under tensile stress, especially in high-strength steels. Stress corrosion cracking involves the combined effect of tensile stress and a corrosive environment, leading to crack propagation along grain boundaries or specific crystallographic planes. Unlike stress corrosion cracking, which requires a corrosive medium, hydrogen embrittlement can occur in non-corrosive environments due to hydrogen introduced during manufacturing or service.
Common Materials Vulnerable to Hydrogen Embrittlement
Hydrogen embrittlement primarily affects high-strength steels, titanium alloys, and certain nickel-based superalloys due to their susceptibility to hydrogen absorption and subsequent loss of ductility. In contrast, stress corrosion cracking often targets stainless steels, aluminum alloys, and copper-based alloys, where specific environmental conditions cause crack propagation. Understanding your material's vulnerability to these mechanisms is crucial for preventing unexpected failures in structural and mechanical components.
Materials Susceptible to Stress Corrosion Cracking
Stress corrosion cracking (SCC) primarily affects austenitic stainless steels, high-strength aluminum alloys, brass, and carbon steels when exposed to specific corrosive environments and tensile stress. Unlike hydrogen embrittlement, which targets high-strength steels and titanium alloys under hydrogen exposure, SCC vulnerability depends on both the material's microstructure and the environmental conditions, such as the presence of chlorides or caustic substances. Understanding which materials are susceptible to stress corrosion cracking helps you select the appropriate alloy and preventive measures to ensure structural integrity in critical applications.
Industry Examples and Real-World Failures
Hydrogen embrittlement frequently compromises high-strength steels in the aerospace and automotive industries, causing catastrophic failures in critical components like landing gear and suspension springs. Stress corrosion cracking predominantly affects stainless steels and superalloys used in chemical processing plants and power generation, with notable incidents such as cracking in nuclear reactor cooling systems and offshore oil platforms. Both mechanisms highlight the necessity for rigorous material selection and environmental control in industries where mechanical stress and corrosive environments converge.
Prevention and Mitigation Strategies
Hydrogen embrittlement prevention relies on reducing hydrogen exposure through protective coatings, controlled environments, and cathodic protection, while stress corrosion cracking (SCC) mitigation emphasizes material selection resistant to specific corrosive agents and stress relief techniques such as heat treatment. Implementing real-time monitoring systems and regular inspection protocols enables early detection and maintenance to prevent crack propagation in both phenomena. Advanced alloy development and surface engineering further enhance resistance, optimizing structural integrity in corrosive and high-stress environments.
Testing and Detection Methods
Hydrogen embrittlement is detected using methods like slow strain rate testing (SSRT), hydrogen permeation tests, and thermal desorption spectroscopy (TDS) to assess hydrogen content and its effects on material brittleness. Stress corrosion cracking (SCC) is commonly identified through tensile testing, fracture surface analysis, and electrochemical tests such as potentiodynamic polarization and electrochemical impedance spectroscopy (EIS) to evaluate crack initiation and propagation under corrosive environments. Non-destructive techniques such as acoustic emission monitoring and ultrasonic testing also aid in early detection of both hydrogen embrittlement and SCC by identifying microcracks and changes in material properties.
Future Trends in Material Protection Against Environmental Cracking
Future trends in material protection against environmental cracking emphasize advanced nanocoatings and smart inhibitors that target hydrogen embrittlement and stress corrosion cracking at the molecular level. Integration of real-time monitoring sensors with AI-driven predictive maintenance systems enhances early detection and mitigation of crack initiation. Research in alloy design focuses on developing corrosion-resistant materials with tailored microstructures to reduce susceptibility to hydrogen-induced damage and stress corrosion mechanisms.
Hydrogen Embrittlement vs Stress Corrosion Cracking Infographic
 libmatt.com
libmatt.com