Blister packs provide individual compartments that protect each tablet or capsule from moisture and contamination, ensuring better product integrity and tamper evidence. Strip packs, often more compact and cost-effective, offer convenience for dosing but may have less protection against external factors, impacting the shelf life of Your medication.
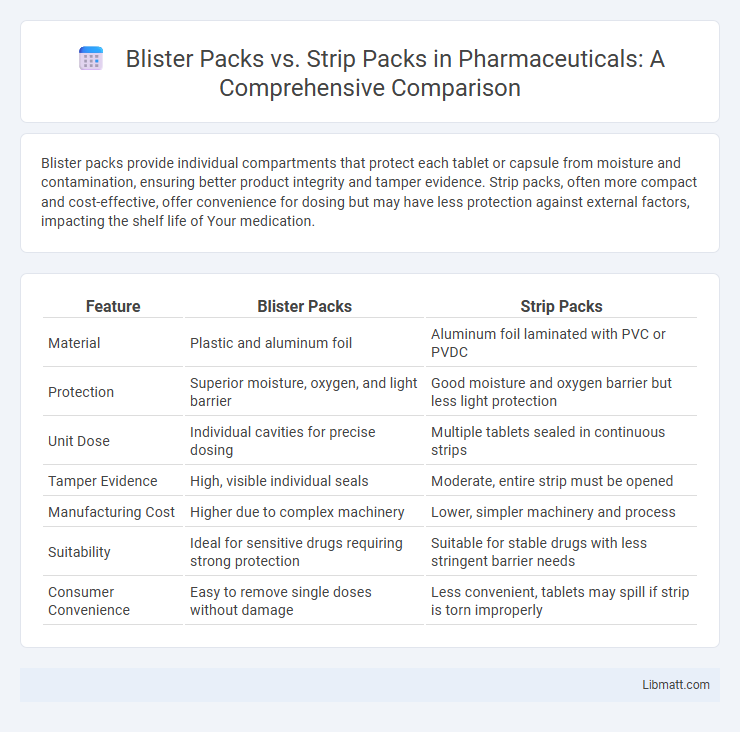
Table of Comparison
| Feature | Blister Packs | Strip Packs |
|---|---|---|
| Material | Plastic and aluminum foil | Aluminum foil laminated with PVC or PVDC |
| Protection | Superior moisture, oxygen, and light barrier | Good moisture and oxygen barrier but less light protection |
| Unit Dose | Individual cavities for precise dosing | Multiple tablets sealed in continuous strips |
| Tamper Evidence | High, visible individual seals | Moderate, entire strip must be opened |
| Manufacturing Cost | Higher due to complex machinery | Lower, simpler machinery and process |
| Suitability | Ideal for sensitive drugs requiring strong protection | Suitable for stable drugs with less stringent barrier needs |
| Consumer Convenience | Easy to remove single doses without damage | Less convenient, tablets may spill if strip is torn improperly |
Introduction to Pharmaceutical Packaging
Blister packs provide individual sealed cavities for each tablet or capsule, offering enhanced protection from moisture, air, and contamination, which extends shelf life and ensures product integrity. Strip packs consist of tablets sealed between a foil and a plastic or paper laminate, allowing for compact, lightweight packaging ideal for high-volume production and easy dispensing. Your choice between blister and strip packs impacts product protection, patient compliance, and manufacturing efficiency in pharmaceutical packaging.
What Are Blister Packs?
Blister packs are packaging solutions consisting of a pre-formed plastic cavity or pocket that securely holds individual doses of medication, protecting them from moisture, contamination, and damage. Commonly made from materials like PVC, PVDC, or aluminum foil, blister packs enable easy patient access while ensuring product stability and extended shelf life. This packaging format is widely used in the pharmaceutical industry for tablets, capsules, and other solid dosage forms, offering clear visibility and tamper-evidence.
What Are Strip Packs?
Strip packs are a type of pharmaceutical packaging where tablets or capsules are sealed between two layers of laminate or foil, providing individual protection to each dose. These packs ensure product integrity by safeguarding against moisture, light, and contamination, making them ideal for maintaining medication efficacy. Choosing strip packs can enhance your medication adherence by offering convenient, portioned doses and tamper-evident packaging.
Key Differences Between Blister and Strip Packs
Blister packs feature a pre-formed plastic cavity sealed with a foil backing, offering superior protection against moisture and contamination compared to strip packs, which consist of tablets sealed between two layers of foil or plastic. Blister packs allow for easy individual tablet removal without compromising the remaining doses, whereas strip packs typically require tearing or cutting, potentially exposing other tablets. Your choice between the two should consider factors like product sensitivity, packaging cost, and patient convenience.
Material Composition and Barrier Properties
Blister packs typically use a combination of plastic materials such as PVC, PET, or PVDC laminated with aluminum foil, providing excellent moisture and oxygen barrier properties essential for protecting sensitive pharmaceuticals. Strip packs are made from layered materials like aluminum foil, paper, and plastic films, offering strong barrier performance but often with less flexibility compared to blister packs. The choice between these packagings depends on product stability requirements, with blister packs favored for superior transparency and barrier efficiency and strip packs preferred for lightweight, cost-effective protection.
Protection and Shelf Life Considerations
Blister packs provide superior protection against moisture, oxygen, and light, significantly enhancing the shelf life of pharmaceuticals by maintaining product integrity and stability. Strip packs, while cost-effective and space-saving, offer less robust barrier properties, making them more suitable for products with shorter shelf life requirements or less sensitivity to environmental factors. Choosing between blister packs and strip packs should consider factors such as product sensitivity, packaging material permeability, and desired shelf life duration to optimize protection and maintain efficacy.
Patient Convenience and Ease of Use
Blister packs offer enhanced patient convenience by providing individually sealed doses that protect medication from contamination and allow easy tracking of daily intake, reducing the risk of missed or double doses. Strip packs, while compact and lightweight, may require patients to peel open the entire strip to access a single dose, potentially causing confusion or spillage. The tactile feedback and clear separation in blister packs make them particularly beneficial for elderly patients or those with limited dexterity.
Manufacturing and Cost Comparison
Blister packs are manufactured by thermoforming plastic sheets into cavities to hold individual doses, offering high product protection but involving higher tooling and material costs, making them suitable for small to medium production runs. Strip packs consist of medicine sealed between layers of aluminum foil and paper or plastic film, produced through simpler, faster processes with lower raw material expenses, which reduce overall cost and enhance scalability for large-scale manufacturing. Choosing between blister and strip packs depends on balancing production volume, budget constraints, and packaging durability requirements in pharmaceutical manufacturing.
Environmental Impact of Packaging Solutions
Blister packs typically generate more plastic waste compared to strip packs, contributing to higher environmental pollution due to their multi-layered materials that are difficult to recycle. Strip packs, often made from less complex materials such as aluminum foil and paper, provide a more eco-friendly option by being easier to separate and recycle. Choosing strip packs over blister packs can significantly reduce the carbon footprint and improve sustainability in pharmaceutical packaging.
Choosing the Right Packaging for Pharmaceuticals
Blister packs offer superior protection against moisture, air, and contamination, ensuring the stability and integrity of individual doses, which is crucial for sensitive pharmaceuticals. Strip packs provide a cost-effective and space-saving solution, ideal for bulk dispensing and ease of use in high-volume settings. Your choice between blister and strip packs should consider factors like drug type, shelf life, patient convenience, and regulatory requirements to optimize safety and effectiveness.
Blister packs vs Strip packs Infographic
 libmatt.com
libmatt.com