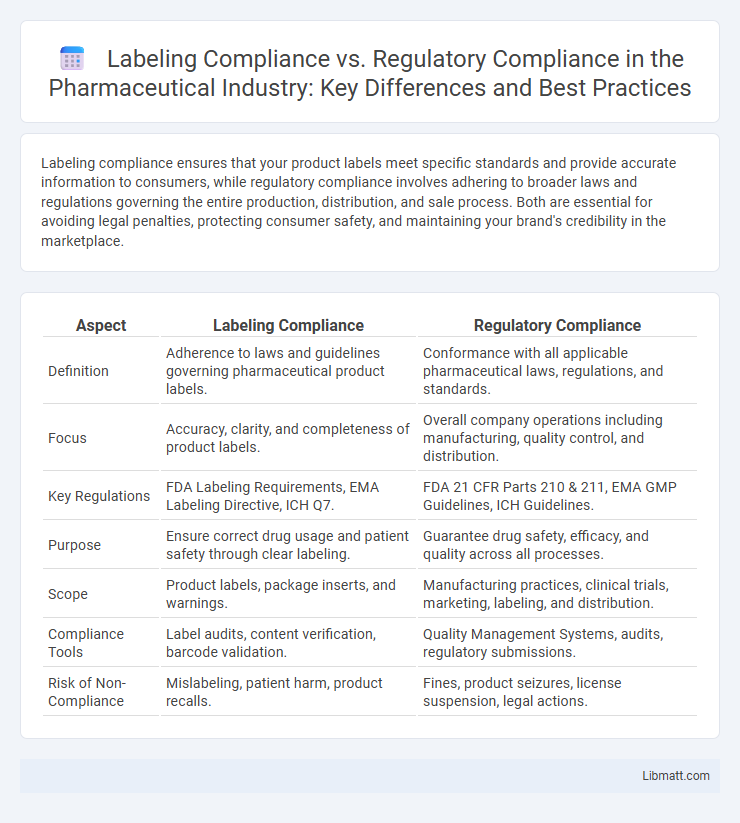
Labeling compliance ensures that your product labels meet specific standards and provide accurate information to consumers, while regulatory compliance involves adhering to broader laws and regulations governing the entire production, distribution, and sale process. Both are essential for avoiding legal penalties, protecting consumer safety, and maintaining your brand's credibility in the marketplace.
Table of Comparison
| Aspect | Labeling Compliance | Regulatory Compliance |
|---|---|---|
| Definition | Adherence to laws and guidelines governing pharmaceutical product labels. | Conformance with all applicable pharmaceutical laws, regulations, and standards. |
| Focus | Accuracy, clarity, and completeness of product labels. | Overall company operations including manufacturing, quality control, and distribution. |
| Key Regulations | FDA Labeling Requirements, EMA Labeling Directive, ICH Q7. | FDA 21 CFR Parts 210 & 211, EMA GMP Guidelines, ICH Guidelines. |
| Purpose | Ensure correct drug usage and patient safety through clear labeling. | Guarantee drug safety, efficacy, and quality across all processes. |
| Scope | Product labels, package inserts, and warnings. | Manufacturing practices, clinical trials, marketing, labeling, and distribution. |
| Compliance Tools | Label audits, content verification, barcode validation. | Quality Management Systems, audits, regulatory submissions. |
| Risk of Non-Compliance | Mislabeling, patient harm, product recalls. | Fines, product seizures, license suspension, legal actions. |
Understanding Labeling Compliance
Labeling compliance ensures product labels meet mandated requirements such as content accuracy, placement, and format to protect consumer safety and provide essential information. It involves adherence to specific laws and standards dictated by agencies like the FDA, EPA, or EU regulations, focusing on ingredient lists, warnings, and usage instructions. Understanding labeling compliance is critical to avoid legal penalties, enhance transparency, and maintain trust within industry-specific frameworks.
Key Aspects of Regulatory Compliance
Regulatory compliance involves adherence to laws, guidelines, and specifications relevant to an industry, ensuring legal and ethical standards are met to avoid penalties and sanctions. Key aspects include understanding applicable regulatory frameworks, implementing proper documentation and reporting procedures, and conducting regular audits to maintain accountability. Organizations must prioritize risk management and employee training to ensure ongoing compliance with evolving regulations and prevent operational disruptions.
Labeling Compliance: Legal Requirements
Labeling compliance involves adhering to specific legal requirements such as accurate ingredient disclosure, allergen warnings, and nutritional information mandated by regulatory bodies like the FDA or EFSA. These regulations ensure product labels meet consumer safety standards and provide truthful information to prevent misleading claims. Failure to comply with labeling laws can result in legal penalties, product recalls, and damage to brand reputation.
Regulatory Compliance: Broader Scope Explained
Regulatory compliance encompasses a wide range of legal requirements set by government agencies to ensure businesses operate within established laws and standards, including safety, environmental, and financial regulations. Labeling compliance is a subset of regulatory compliance, focused specifically on the accuracy, clarity, and legality of product labels to protect consumers and facilitate market transparency. Understanding your obligations in regulatory compliance helps avoid legal penalties and ensures your business meets all applicable industry standards.
Differences Between Labeling and Regulatory Compliance
Labeling compliance ensures that product labels accurately reflect contents, instructions, and safety warnings to meet industry standards, while regulatory compliance involves adhering to broader laws and regulations governing product manufacturing, distribution, and marketing. Labeling compliance is a component of regulatory compliance but is more focused on consumer information and specific product presentation. Your business must address both to avoid legal penalties and ensure market access across different jurisdictions.
Importance of Accurate Product Labeling
Accurate product labeling plays a critical role in both labeling compliance and regulatory compliance, ensuring that all product information meets legal standards and protects consumer safety. Mislabeling can result in severe penalties, product recalls, and damage to brand reputation, emphasizing the necessity for meticulous verification processes. Companies must adhere strictly to guidelines set by regulatory bodies such as the FDA or EPA to maintain market access and avoid legal liabilities.
Regulatory Frameworks Affecting Labeling
Regulatory frameworks affecting labeling include laws such as the FDA's Food Labeling Guide, the EU's FIC Regulation, and ISO standards that dictate accurate product information to ensure consumer safety. Labeling compliance requires adherence to specific regulations on content, format, and language, while regulatory compliance encompasses a broader scope including manufacturing practices and safety standards. Ensuring your product meets these labeling regulations prevents legal risks and enhances market trust.
Common Challenges in Compliance Management
Labeling compliance and regulatory compliance both face challenges such as keeping up with evolving legislation, managing accurate documentation, and ensuring consistent implementation across multiple regions. Your team must navigate complex standards and frequent updates that can lead to errors or delays, increasing the risk of penalties and product recalls. Efficient compliance management requires robust tracking systems and expert knowledge to address discrepancies in label requirements and regulatory mandates effectively.
Consequences of Non-Compliance
Non-compliance with labeling regulations can lead to product recalls, legal penalties, and damage to Your brand's reputation, directly impacting consumer trust and sales. Regulatory compliance ensures adherence to safety, environmental, and industry-specific laws, preventing costly fines and operational shutdowns. Both labeling and regulatory non-compliance increase liability risks and hinder market access, emphasizing the importance of strict compliance management.
Best Practices for Achieving Full Compliance
Achieving full compliance requires integrating labeling compliance with regulatory compliance by adhering strictly to industry standards, such as FDA, OSHA, or ISO guidelines, ensuring all product labels accurately reflect mandatory safety, ingredient, and usage information. Implementing robust quality control systems and conducting regular audits mitigate risks of mislabeling and non-compliance penalties. Leveraging digital tools for label management streamlines updates to regulatory changes, maintaining alignment with evolving legal requirements and enhancing traceability.
Labeling compliance vs regulatory compliance Infographic
 libmatt.com
libmatt.com