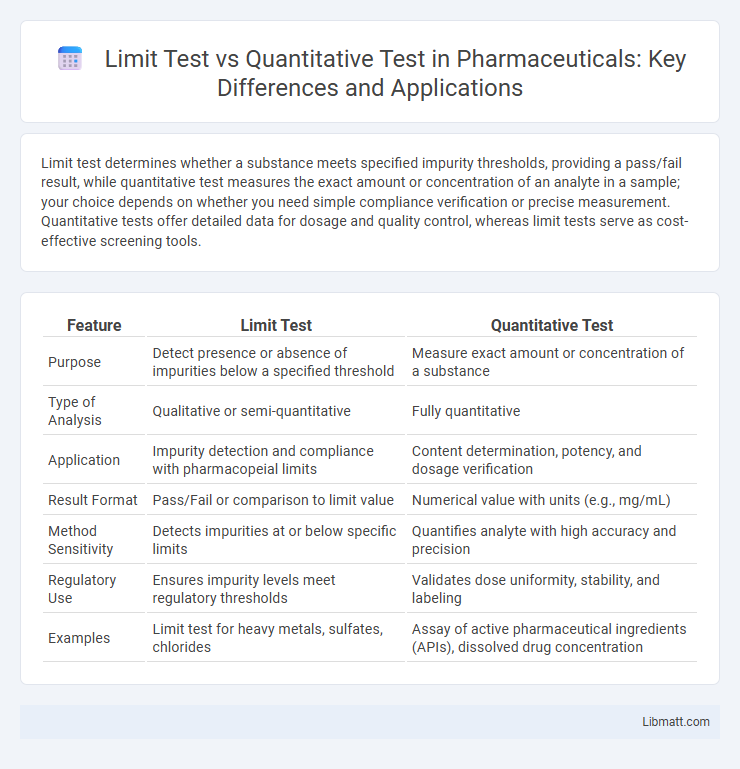
Limit test determines whether a substance meets specified impurity thresholds, providing a pass/fail result, while quantitative test measures the exact amount or concentration of an analyte in a sample; your choice depends on whether you need simple compliance verification or precise measurement. Quantitative tests offer detailed data for dosage and quality control, whereas limit tests serve as cost-effective screening tools.
Table of Comparison
| Feature | Limit Test | Quantitative Test |
|---|---|---|
| Purpose | Detect presence or absence of impurities below a specified threshold | Measure exact amount or concentration of a substance |
| Type of Analysis | Qualitative or semi-quantitative | Fully quantitative |
| Application | Impurity detection and compliance with pharmacopeial limits | Content determination, potency, and dosage verification |
| Result Format | Pass/Fail or comparison to limit value | Numerical value with units (e.g., mg/mL) |
| Method Sensitivity | Detects impurities at or below specific limits | Quantifies analyte with high accuracy and precision |
| Regulatory Use | Ensures impurity levels meet regulatory thresholds | Validates dose uniformity, stability, and labeling |
| Examples | Limit test for heavy metals, sulfates, chlorides | Assay of active pharmaceutical ingredients (APIs), dissolved drug concentration |
Understanding Limit Test in Analytical Chemistry
Limit tests in analytical chemistry are qualitative procedures designed to detect the presence or absence of impurities up to a specified maximum concentration, ensuring safety and compliance. Quantitative tests, by contrast, measure the exact amount of a substance present, providing precise concentration values. You rely on limit tests to quickly verify that impurity levels do not exceed regulatory thresholds.
What is a Quantitative Test?
A quantitative test measures the precise amount or concentration of a specific substance within a sample, providing exact numerical data essential for quality control and compliance with regulatory standards. Unlike limit tests that merely indicate whether a substance is present above or below a certain threshold, quantitative tests deliver detailed, measurable results critical for dosage accuracy and product consistency. You rely on quantitative testing to ensure formulation accuracy and maintain the safety and efficacy of pharmaceuticals or chemical products.
Key Differences Between Limit Test and Quantitative Test
Limit tests determine the presence or absence of impurities in a sample by comparing them against a predefined threshold, ensuring safety and compliance without measuring the exact quantity. Quantitative tests measure the precise amount or concentration of a substance within a sample, providing detailed numerical data essential for dosage and formulation accuracy. The key difference lies in limit tests offering qualitative pass/fail results, whereas quantitative tests deliver exact measurements crucial for quality control and regulatory approval.
Principles Underlying Limit Test Methods
Limit test methods rely on qualitative observation to detect whether a substance's impurity or component concentration exceeds a predetermined threshold. These tests use standardized reagents and color changes or precipitate formation to indicate presence at or above the set limit, ensuring a rapid and simple initial assessment. Unlike quantitative tests, which measure exact concentrations, limit tests prioritize safety by confirming that potentially harmful levels do not surpass regulatory limits, providing a practical approach for You in quality control processes.
Principles and Techniques in Quantitative Testing
Limit tests determine the presence of impurities by comparing sample concentration against predefined thresholds using qualitative methods, whereas quantitative tests measure the exact amount of an analyte through precise techniques such as titration, spectrophotometry, or chromatography. Principles in quantitative testing rely on accurate measurement of analyte concentration by applying calibration curves, stoichiometric reactions, or instrument response factors to ensure reproducibility and sensitivity. Your ability to select appropriate quantitative techniques depends on understanding sample nature, required accuracy, and analytical instrumentation capabilities.
Applications of Limit Test in Pharmaceutical Analysis
Limit tests in pharmaceutical analysis primarily detect and control trace amounts of impurities or contaminants to ensure drug safety and compliance with regulatory standards. These tests are widely applied for detecting heavy metals, chloride, sulfate, and other specific ions within acceptable limits in raw materials and finished products. Your pharmaceutical quality control process benefits from limit tests by providing a rapid, cost-effective method to verify product purity without requiring extensive quantitative analysis.
Importance of Quantitative Tests in Quality Control
Quantitative tests provide precise measurements of substance concentrations, essential for ensuring product consistency and compliance with regulatory standards in quality control. These tests enable accurate detection of active ingredients, contaminants, and impurities, directly impacting the safety and efficacy of pharmaceuticals and other products. Your quality control processes rely on quantitative data to maintain product integrity and meet industry specifications effectively.
Sensitivity and Specificity: Limit Test vs Quantitative Test
Limit tests offer high specificity by detecting the presence or absence of an impurity above a predefined threshold, making them ideal for screening purposes. Quantitative tests provide enhanced sensitivity by measuring the exact concentration of substances, enabling precise determination of compliance with regulatory limits. Your choice between these methods depends on whether you require a simple pass/fail result or detailed analytical data for accurate quality control.
Common Examples: Limit Test and Quantitative Test Procedures
Limit tests commonly include chloride, sulfate, and heavy metals tests, designed to determine if the presence of specific impurities exceeds predefined acceptable levels in pharmaceutical substances. Quantitative test procedures involve precise measurement techniques such as titration, spectrophotometry, and chromatography to accurately quantify the concentration of active ingredients or contaminants. Both testing methods ensure compliance with pharmacopeial standards, with limit tests serving as qualitative or semi-quantitative checks and quantitative tests providing exact numerical results.
Choosing the Right Test: Factors to Consider
Choosing the right test between limit test and quantitative test depends on your specific regulatory requirements, the precision needed, and the nature of the substance being analyzed. Limit tests provide a pass/fail outcome for impurities within defined thresholds, ideal for compliance with pharmacopeial standards, while quantitative tests offer exact measurement of analyte concentration, essential for dosage precision and quality control. Assess sample complexity, testing purpose, and available instrumentation to determine the most effective approach for your analysis.
Limit test vs quantitative test Infographic
 libmatt.com
libmatt.com