Normal Phase HPLC separates compounds based on polarity using a polar stationary phase and a non-polar mobile phase, ideal for analyzing polar compounds. Reverse Phase HPLC utilizes a non-polar stationary phase with a polar mobile phase, making it more versatile for separating a broad range of non-polar to moderately polar substances.
Table of Comparison
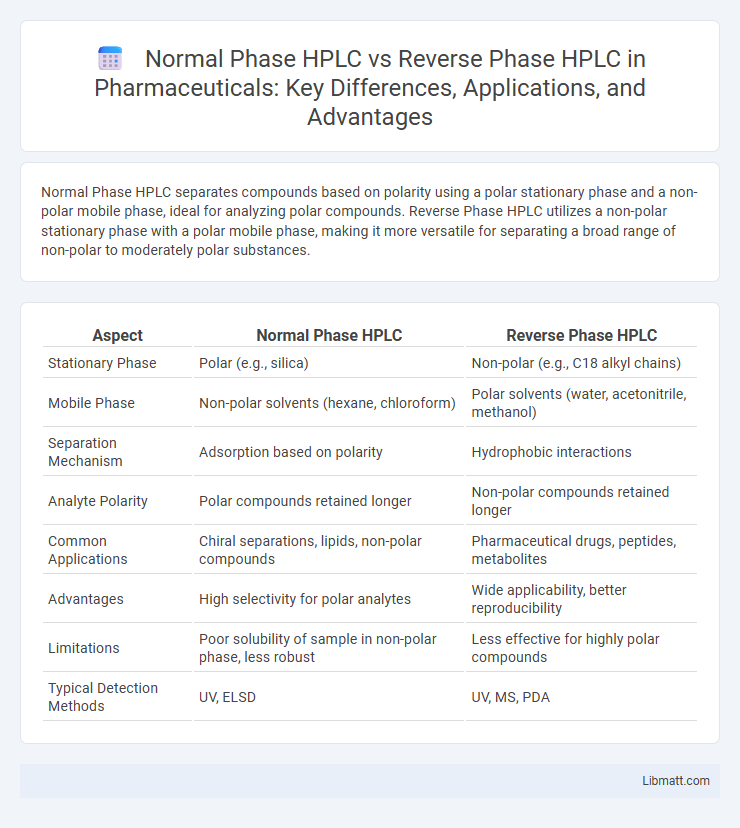
| Aspect | Normal Phase HPLC | Reverse Phase HPLC |
|---|---|---|
| Stationary Phase | Polar (e.g., silica) | Non-polar (e.g., C18 alkyl chains) |
| Mobile Phase | Non-polar solvents (hexane, chloroform) | Polar solvents (water, acetonitrile, methanol) |
| Separation Mechanism | Adsorption based on polarity | Hydrophobic interactions |
| Analyte Polarity | Polar compounds retained longer | Non-polar compounds retained longer |
| Common Applications | Chiral separations, lipids, non-polar compounds | Pharmaceutical drugs, peptides, metabolites |
| Advantages | High selectivity for polar analytes | Wide applicability, better reproducibility |
| Limitations | Poor solubility of sample in non-polar phase, less robust | Less effective for highly polar compounds |
| Typical Detection Methods | UV, ELSD | UV, MS, PDA |
Introduction to HPLC Techniques
Normal Phase HPLC employs a polar stationary phase and non-polar mobile phase, making it ideal for separating compounds based on polarity differences where polar analytes have stronger retention. Reverse Phase HPLC uses a non-polar stationary phase with a polar mobile phase, favoring the retention of hydrophobic compounds and enabling high reproducibility and versatility in pharmaceutical and biochemical analysis. The choice between these techniques depends on the chemical nature of the sample and desired separation outcomes.
Overview of Normal Phase HPLC
Normal Phase HPLC utilizes a polar stationary phase and a non-polar mobile phase, making it ideal for separating compounds based on polarity differences. This technique excels in analyzing polar compounds, such as lipids, steroids, and pharmaceuticals, by retaining them longer on the polar stationary phase. Your choice of Normal Phase HPLC benefits applications requiring high-resolution separations of hydrophilic molecules that are less effectively separated by Reverse Phase HPLC.
Overview of Reverse Phase HPLC
Reverse Phase HPLC utilizes a non-polar stationary phase paired with a polar mobile phase, making it ideal for separating compounds based on hydrophobic interactions. Your analysis benefits from its high reproducibility and versatility in handling a wide range of sample polarities, especially pharmaceuticals and biomolecules. This method excels in providing sharp peaks and high resolution, crucial for precise quantitative and qualitative assessments.
Principle of Separation in Normal Phase
Normal Phase HPLC separates compounds based on their polarity using a polar stationary phase and a non-polar mobile phase, where polar analytes interact strongly with the stationary phase and elute slower. The separation principle relies on differential adsorption affinities, with less polar compounds eluting first due to weaker interactions with the stationary phase. This contrasts with Reverse Phase HPLC, which uses a non-polar stationary phase and polar mobile phase, separating analytes primarily based on hydrophobic interactions.
Principle of Separation in Reverse Phase
Reverse Phase HPLC separates compounds based on hydrophobic interactions between the non-polar stationary phase and the analytes, causing more hydrophobic molecules to elute later. Your sample components partition between the polar mobile phase and the non-polar stationary phase, optimizing separation by varying the mobile phase's polarity. This principle contrasts with Normal Phase HPLC, which utilizes polar stationary phases and non-polar mobile phases for separation based on polarity differences.
Key Differences Between Normal and Reverse Phase HPLC
Normal Phase HPLC uses a polar stationary phase and a non-polar mobile phase, making it ideal for separating hydrophilic compounds, whereas Reverse Phase HPLC employs a non-polar stationary phase with a polar mobile phase, favoring the separation of hydrophobic molecules. The retention mechanism in Normal Phase HPLC relies on adsorption interactions, while Reverse Phase HPLC depends primarily on hydrophobic interactions between analytes and the stationary phase. Your choice between these methods depends on the polarity of the analytes and the desired resolution efficiency within your chromatographic analysis.
Applications of Normal Phase HPLC
Normal Phase HPLC is widely used for separating non-polar compounds, chiral molecules, and lipophilic substances that are poorly retained in Reverse Phase HPLC. Its key applications include the analysis of pharmaceuticals, natural products, and complex mixtures containing isomers or stereoisomers. You can benefit from Normal Phase HPLC when working with compounds that require interaction with polar stationary phases for optimal resolution.
Applications of Reverse Phase HPLC
Reverse Phase HPLC is extensively used for the separation and analysis of non-polar to moderately polar compounds, including pharmaceuticals, peptides, and environmental samples. Its compatibility with aqueous mobile phases makes it ideal for drug purity testing, biomolecule analysis, and food safety monitoring. You can rely on Reverse Phase HPLC for high reproducibility and sensitivity in complex mixture analysis, unlike Normal Phase HPLC which is better suited for non-polar solvents and less polar analytes.
Advantages and Limitations of Each Method
Normal Phase HPLC offers superior separation of non-polar compounds and is highly effective for analyzing polar analytes due to its polar stationary phase, but it requires non-polar solvents and may suffer from longer equilibration times and limited solvent compatibility. Reverse Phase HPLC excels in robustness and versatility, utilizing a non-polar stationary phase and polar aqueous mobile phases that are compatible with a wide range of samples and detectors, yet it may struggle with highly polar compounds and can produce less retention for hydrophilic molecules. Your choice between these methods depends on the compound polarity, sample complexity, and solvent system preferences to optimize analytical performance.
Choosing the Right HPLC Method for Your Analysis
Selecting the appropriate HPLC method depends on the polarity of the analytes and stationary phases; Normal Phase HPLC utilizes a polar stationary phase and non-polar mobile phase, ideal for separating hydrophilic compounds. Reverse Phase HPLC employs a non-polar stationary phase with a polar mobile phase, offering high reproducibility and broad applicability for hydrophobic molecules. Consider sample solubility, analyte polarity, and desired resolution to optimize separation efficiency and analytical accuracy.
Normal Phase HPLC vs Reverse Phase HPLC Infographic
 libmatt.com
libmatt.com