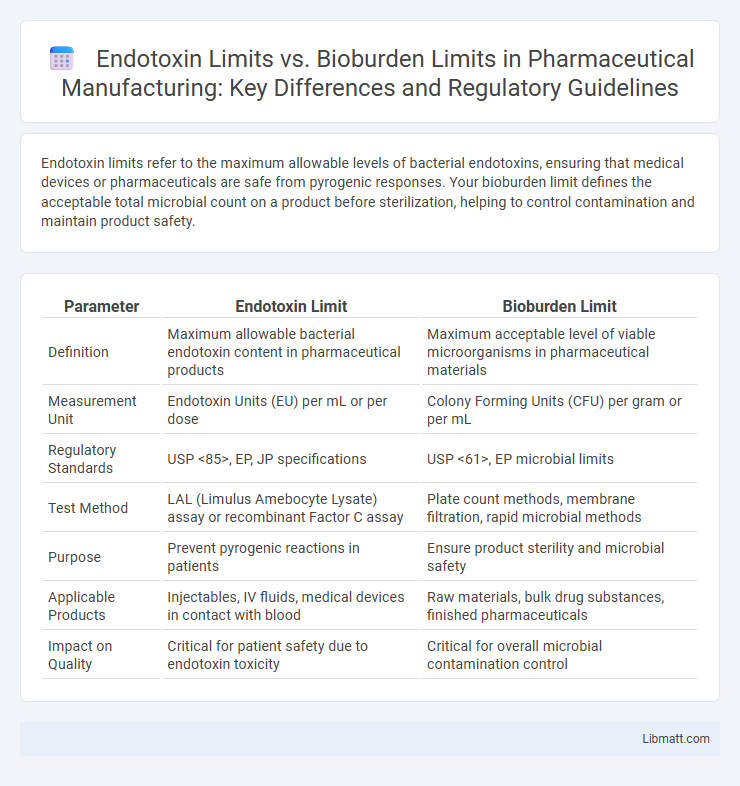
Endotoxin limits refer to the maximum allowable levels of bacterial endotoxins, ensuring that medical devices or pharmaceuticals are safe from pyrogenic responses. Your bioburden limit defines the acceptable total microbial count on a product before sterilization, helping to control contamination and maintain product safety.
Table of Comparison
| Parameter | Endotoxin Limit | Bioburden Limit |
|---|---|---|
| Definition | Maximum allowable bacterial endotoxin content in pharmaceutical products | Maximum acceptable level of viable microorganisms in pharmaceutical materials |
| Measurement Unit | Endotoxin Units (EU) per mL or per dose | Colony Forming Units (CFU) per gram or per mL |
| Regulatory Standards | USP <85>, EP, JP specifications | USP <61>, EP microbial limits |
| Test Method | LAL (Limulus Amebocyte Lysate) assay or recombinant Factor C assay | Plate count methods, membrane filtration, rapid microbial methods |
| Purpose | Prevent pyrogenic reactions in patients | Ensure product sterility and microbial safety |
| Applicable Products | Injectables, IV fluids, medical devices in contact with blood | Raw materials, bulk drug substances, finished pharmaceuticals |
| Impact on Quality | Critical for patient safety due to endotoxin toxicity | Critical for overall microbial contamination control |
Understanding Endotoxin Limit: Definition and Importance
Endotoxin Limit defines the maximum allowable concentration of bacterial endotoxins in pharmaceutical products to ensure patient safety and prevent adverse reactions such as fever or inflammation. This threshold is critical in sterile drug manufacturing, where endotoxin contamination can originate from gram-negative bacteria and must be minimized through rigorous testing and control measures. Understanding Your product's Endotoxin Limit guides quality control protocols and compliance with regulatory standards like those from the FDA and USP.
What is Bioburden Limit? A Comprehensive Overview
Bioburden Limit refers to the maximum allowable level of microbial contamination on a product or within a manufacturing environment before sterilization. It ensures that the microbial load is controlled to prevent product spoilage or infection risks, directly impacting your sterilization process and quality assurance. Setting an appropriate Bioburden Limit is essential for compliance with regulatory standards and maintaining product safety.
Regulatory Standards for Endotoxin and Bioburden Limits
Regulatory standards for endotoxin and bioburden limits are defined by agencies such as the FDA, USP, and EMA, requiring strict adherence to ensure patient safety in pharmaceutical products. Endotoxin limits are typically established based on product type and maximum recommended dosage, with USP <85> setting specific endotoxin thresholds measured in endotoxin units (EU). Bioburden limits are influenced by product sterility requirements and manufacturing processes, with USP <61> and <62> providing guidelines for microbial counts to prevent contamination and maintain product quality.
Key Differences Between Endotoxin and Bioburden Limits
Endotoxin limits refer to the maximum allowable amount of bacterial toxins, measured in endotoxin units (EU), crucial for preventing pyrogenic reactions in parenteral drugs, while bioburden limits specify the acceptable levels of viable microorganisms present during manufacturing to ensure overall microbial quality. The endotoxin limit primarily targets non-viable toxin contamination from Gram-negative bacteria, whereas bioburden limits address live microbial contamination from various sources impacting sterility assurance. Understanding these key differences helps you establish appropriate quality control measures to comply with regulatory standards like USP <85> for endotoxins and USP <61> for bioburden testing.
Methods for Determining Endotoxin Levels
Endotoxin levels are primarily determined using the Limulus Amebocyte Lysate (LAL) assay, which detects bacterial endotoxins by catalyzing a clotting reaction in horseshoe crab blood cells. Alternative methods include the recombinant Factor C assay, a synthetic and more ethical approach, and the gel-clot, turbidimetric, and chromogenic techniques, each providing varying sensitivity and quantification capabilities. Rigorous validation of these methods ensures compliance with pharmacopeial guidelines for endotoxin limits, distinguishing them from bioburden testing that quantifies viable microbial contamination.
Techniques for Measuring Bioburden
Techniques for measuring bioburden include plate count methods, membrane filtration, and rapid microbiological methods like ATP bioluminescence and flow cytometry. These techniques quantify viable microorganisms present in a sample, helping ensure compliance with bioburden limits critical for product safety. Your selection of method depends on sample type, required sensitivity, and regulatory standards to maintain endotoxin and bioburden control effectively.
Impact of Exceeding Endotoxin and Bioburden Limits
Exceeding endotoxin limits can cause severe inflammatory responses, including fever, shock, and organ failure, posing significant risks for patient safety and product sterility. Surpassing bioburden limits compromises the sterility assurance level, increasing the likelihood of microbial contamination and potential product recalls or regulatory non-compliance. Both exceedances undermine pharmaceutical quality control, necessitating strict monitoring and mitigation strategies to ensure compliance with pharmacopeial standards.
Endotoxin vs Bioburden: Risk Assessment in Pharmaceuticals
Endotoxin limit and bioburden limit are critical parameters in pharmaceutical risk assessment, where endotoxin refers to lipopolysaccharides from gram-negative bacteria posing pyrogenic risks, while bioburden represents the total viable microbial load on a product or surface. Controlling endotoxin levels is essential to prevent febrile reactions in parenteral products, with specific limits established based on the product's maximum human dose and administration route. Bioburden control helps minimize the risk of microbial contamination, reducing endotoxin formation by controlling upstream microbial growth and ensuring sterility assurance in manufacturing processes.
Strategies for Controlling Endotoxin and Bioburden
Effective strategies for controlling endotoxin and bioburden limits include rigorous environmental monitoring and validated sterilization processes to minimize microbial contamination. Implementing strict raw material controls and routine testing ensures endotoxin levels remain within acceptable thresholds, protecting the safety of your pharmaceutical products. Continuous employee training and adherence to GMP guidelines further reduce the risk of contamination and maintain compliance with regulatory standards.
Best Practices for Compliance with Regulatory Limits
Maintaining adherence to endotoxin and bioburden limits requires stringent monitoring of manufacturing processes and environmental controls to prevent contamination. Implementing validated cleaning procedures, routine microbial testing, and endotoxin assays ensures your products consistently meet regulatory standards. Utilizing statistical process control and maintaining comprehensive documentation further supports compliance and facilitates regulatory inspections.
Endotoxin Limit vs Bioburden Limit Infographic
 libmatt.com
libmatt.com