Off-label use refers to prescribing medications for an unapproved indication, dosage, or patient population while the drug remains licensed, whereas unlicensed use involves administering drugs that have not received regulatory approval for any indication. Your healthcare provider carefully considers the risks and benefits before recommending either off-label or unlicensed treatments to ensure safety and efficacy.
Table of Comparison
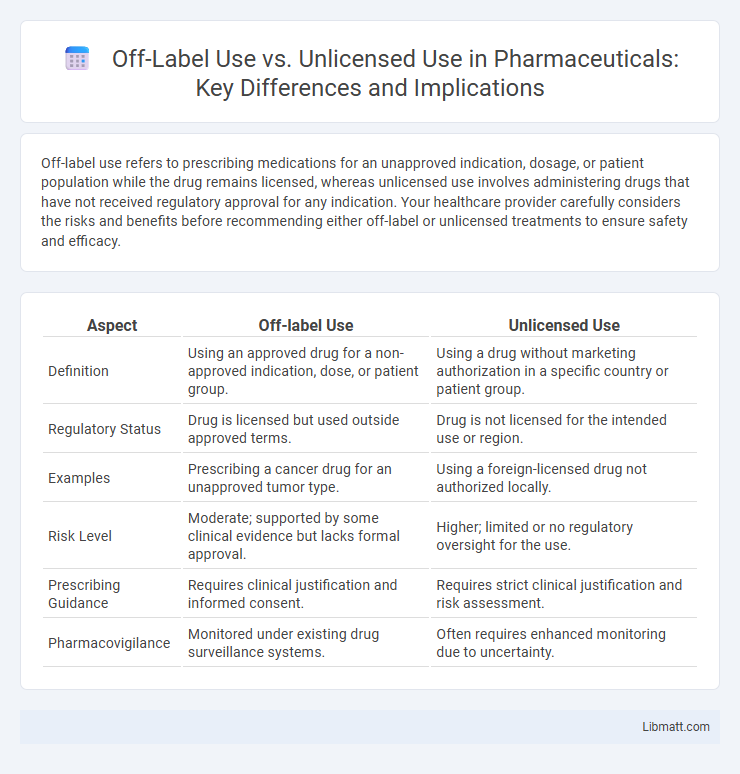
| Aspect | Off-label Use | Unlicensed Use |
|---|---|---|
| Definition | Using an approved drug for a non-approved indication, dose, or patient group. | Using a drug without marketing authorization in a specific country or patient group. |
| Regulatory Status | Drug is licensed but used outside approved terms. | Drug is not licensed for the intended use or region. |
| Examples | Prescribing a cancer drug for an unapproved tumor type. | Using a foreign-licensed drug not authorized locally. |
| Risk Level | Moderate; supported by some clinical evidence but lacks formal approval. | Higher; limited or no regulatory oversight for the use. |
| Prescribing Guidance | Requires clinical justification and informed consent. | Requires strict clinical justification and risk assessment. |
| Pharmacovigilance | Monitored under existing drug surveillance systems. | Often requires enhanced monitoring due to uncertainty. |
Understanding Off-Label and Unlicensed Drug Use
Off-label use refers to prescribing an approved medication for a different condition, dose, or patient group than officially authorized, while unlicensed use involves medications not approved by regulatory authorities for any indication. Understanding these distinctions is crucial for ensuring safe, effective treatment and compliance with legal standards. Your awareness of off-label and unlicensed drug use helps optimize patient care while mitigating potential risks associated with unintended medication use.
Key Definitions: Off-Label vs Unlicensed Use
Off-label use refers to prescribing a medication for an indication, dosage, or patient group not approved by regulatory agencies, while unlicensed use involves utilizing drugs that lack formal market authorization for any indication. Off-label prescribing relies on existing licensed medications beyond their approved specifications, whereas unlicensed use includes importing, compounding, or modifying drugs without official registration. Understanding the distinction helps ensure your treatment aligns with safety standards and legal considerations.
Regulatory Perspectives on Drug Use
Regulatory perspectives distinguish off-label use as prescribing an approved drug for an unapproved indication, dosage, or population within the scope of licensed products, subject to physician discretion but without regulatory approval for that specific use. Unlicensed use involves prescribing drugs not authorized by regulatory agencies for any indication, often requiring special authorizations or compassionate use protocols to ensure patient safety and legal compliance. Regulatory frameworks emphasize rigorous evaluation and monitoring of off-label and unlicensed drug use to mitigate risks, promote informed consent, and uphold pharmacovigilance standards.
Clinical Justifications for Off-Label Prescribing
Off-label prescribing occurs when clinicians use approved medications for indications, dosages, or patient groups not specified in the official labeling, often driven by emerging evidence or clinical necessity. Clinical justifications include addressing unmet medical needs, rare diseases, or pediatric cases where approved treatments are limited or unavailable. When you consider off-label use, thorough evaluation of safety, efficacy, and ethical implications ensures that patient care remains evidence-based and tailored.
Risks and Benefits of Unlicensed Medications
Unlicensed medications carry significant risks, including unknown safety profiles, potential adverse reactions, and lack of rigorous testing or regulatory approval, which can compromise patient health. However, the benefits may include access to treatments when no licensed options exist, offering potential therapeutic relief for rare or complex conditions. Your healthcare provider must carefully weigh these factors, ensuring that the potential advantages outweigh the risks before recommending unlicensed drug use.
Legal Implications for Healthcare Providers
Healthcare providers face significant legal implications when prescribing off-label or unlicensed medications, as off-label use involves approved drugs used in a manner not specified by regulatory agencies, while unlicensed use pertains to drugs without formal authorization for any indication. Off-label prescribing may result in increased liability if adverse outcomes occur, requiring thorough documentation and informed consent to mitigate risks. Unlicensed drug use carries higher legal risks due to the absence of regulatory approval, demanding rigorous justification and adherence to institutional protocols to protect both patients and providers.
Impact on Patient Safety and Outcomes
Off-label use involves prescribing an approved medication for an unapproved indication, dosage, or population, which can result in uncertain safety and efficacy profiles, potentially increasing the risk of adverse drug reactions and suboptimal therapeutic outcomes. Unlicensed use refers to administering medicines without regulatory approval, often leading to a lack of standardized dosing guidelines and monitoring protocols, thereby raising concerns about patient safety and inconsistent clinical results. Both practices require rigorous clinical judgment and informed consent to mitigate risks and optimize patient care while addressing gaps in evidence-based treatments.
Pharmaceutical Industry and Off-Label Promotion
The pharmaceutical industry often navigates complex regulations surrounding off-label use, where medications are prescribed for indications, dosages, or populations not explicitly approved by regulatory agencies like the FDA or EMA. Off-label promotion by pharmaceutical companies is strictly prohibited in many jurisdictions to prevent misleading claims and ensure patient safety, as it can lead to unapproved drug uses lacking rigorous evidence. Unlicensed use, involving drugs without marketing authorization, also raises legal and ethical concerns, highlighting the need for clear guidelines and compliance to maintain trust and uphold public health standards.
Ethical Considerations in Off-Label and Unlicensed Use
Ethical considerations in off-label and unlicensed drug use revolve around informed consent, patient safety, and evidence-based practice. Healthcare professionals must balance the potential benefits against risks, ensuring transparency and clear communication with patients regarding the lack of formal approval for specific indications or populations. Rigorous documentation and ongoing monitoring are essential to uphold ethical standards and optimize clinical outcomes.
Best Practices for Prescribing and Monitoring
Best practices for prescribing off-label or unlicensed medications include thorough documentation of the clinical rationale and obtaining informed consent from patients. Healthcare providers should closely monitor therapeutic outcomes and adverse reactions, employing standardized assessment tools to ensure patient safety. Collaboration with multidisciplinary teams and regular review of emerging evidence support optimized treatment decisions in these contexts.
Off-label use vs Unlicensed use Infographic
 libmatt.com
libmatt.com