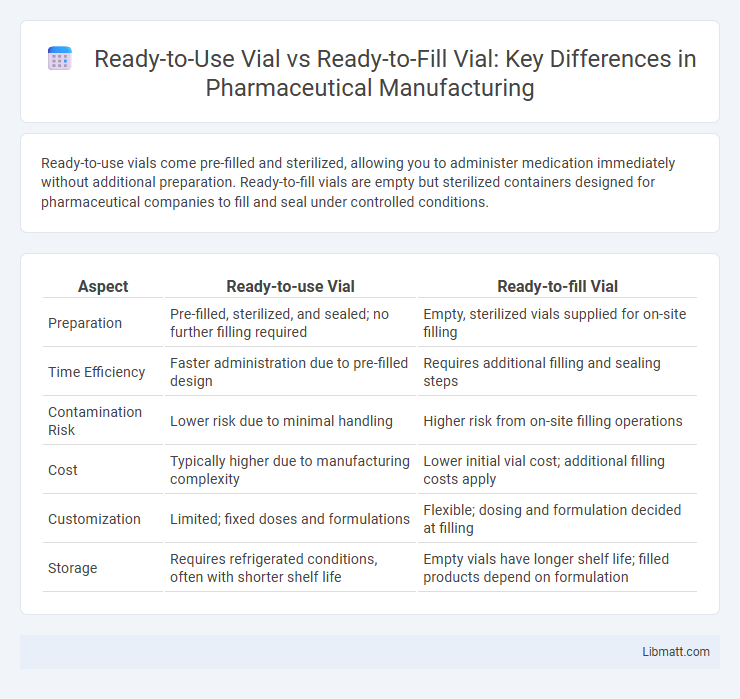
Ready-to-use vials come pre-filled and sterilized, allowing you to administer medication immediately without additional preparation. Ready-to-fill vials are empty but sterilized containers designed for pharmaceutical companies to fill and seal under controlled conditions.
Table of Comparison
| Aspect | Ready-to-use Vial | Ready-to-fill Vial |
|---|---|---|
| Preparation | Pre-filled, sterilized, and sealed; no further filling required | Empty, sterilized vials supplied for on-site filling |
| Time Efficiency | Faster administration due to pre-filled design | Requires additional filling and sealing steps |
| Contamination Risk | Lower risk due to minimal handling | Higher risk from on-site filling operations |
| Cost | Typically higher due to manufacturing complexity | Lower initial vial cost; additional filling costs apply |
| Customization | Limited; fixed doses and formulations | Flexible; dosing and formulation decided at filling |
| Storage | Requires refrigerated conditions, often with shorter shelf life | Empty vials have longer shelf life; filled products depend on formulation |
Introduction to Pharmaceutical Vials
Pharmaceutical vials serve as critical containers for sterile drugs, with ready-to-use vials offered pre-sterilized and sealed, enabling immediate drug filling without further preparation. Ready-to-fill vials arrive non-sterile, requiring sterilization within manufacturing facilities before drug compounding, allowing for greater flexibility in customization and batch sizes. Selection between ready-to-use and ready-to-fill vials depends on factors such as production scale, sterilization capabilities, and regulatory compliance in pharmaceutical manufacturing.
What is a Ready-to-use (RTU) Vial?
A Ready-to-use (RTU) vial is a pre-sterilized container designed for immediate use in pharmaceutical and biotechnology applications, eliminating the need for additional preparation steps. This vial enhances operational efficiency by reducing the risk of contamination and ensures consistent quality control, making it ideal for direct drug filling or administration. Your production process benefits from streamlined workflows and minimized downtime when utilizing RTU vials.
What is a Ready-to-fill (RTF) Vial?
A Ready-to-fill (RTF) vial is a sterile, depyrogenated container designed for pharmaceutical manufacturing that requires no additional washing or sterilization before filling. These vials streamline your production process by reducing contamination risks and saving time compared to bulk vials that need in-house preparation. RTF vials come with integrated features such as blow-back or bottom blow, enhancing automated filling line compatibility.
Key Differences Between RTU and RTF Vials
Ready-to-use (RTU) vials come pre-filled and sealed by the manufacturer, ensuring immediate usability with minimal preparation, which reduces contamination risks and speeds up administration. Ready-to-fill (RTF) vials, however, are supplied as sterilized, empty containers designed for aseptic filling in cleanroom environments, offering greater flexibility in dosage and formulation customization. Understanding these distinctions helps you select the appropriate vial type based on manufacturing workflow efficiency, regulatory protocols, and pharmaceutical packaging requirements.
Advantages of Ready-to-use Vials
Ready-to-use vials offer significant advantages including enhanced sterility assurance since they come pre-sterilized and sealed, reducing contamination risks during handling. They streamline the pharmaceutical filling process by eliminating the need for in-house sterilization and depyrogenation, saving time and operational costs. Additionally, ready-to-use vials ensure consistent quality and compliance with regulatory standards, improving overall production efficiency.
Benefits of Ready-to-fill Vials
Ready-to-fill vials offer significant advantages in pharmaceutical manufacturing, including reduced contamination risk due to their sterile, ready-to-use condition. These vials streamline the filling process by eliminating the need for washing and depyrogenation, resulting in increased production efficiency and cost savings. High-quality materials and validated sterility further ensure product safety and regulatory compliance in aseptic processing environments.
Manufacturing Process Comparison
Ready-to-use vials undergo a complete manufacturing process including sterilization, filling, and sealing in controlled environments before distribution, ensuring immediate usability. Ready-to-fill vials, by contrast, are produced and sterilized but require downstream filling and sealing operations at the pharmaceutical facility, offering flexibility but demanding additional cleanroom resources. Your choice between the two impacts production timelines, contamination risk management, and operational efficiency within your drug manufacturing workflow.
Impact on Sterility and Quality Assurance
Ready-to-use vials offer enhanced sterility by minimizing manual handling and reducing contamination risks during the filling process, ensuring consistent quality assurance. In contrast, ready-to-fill vials require aseptic filling environments and stringent in-house sterilization protocols to maintain sterility, posing higher contamination challenges. Implementing validated sterilization methods and rigorous environmental controls is critical for ready-to-fill vials to meet quality assurance standards equivalent to ready-to-use alternatives.
Cost Implications: RTU vs. RTF Vials
Ready-to-use (RTU) vials typically have higher upfront costs due to pre-sterilization and quality assurance processes, but they reduce labor and contamination risks during production. Ready-to-fill (RTF) vials offer lower initial costs by requiring sterilization and filling steps in-house, which can increase operational expenses and complexity. Choosing between RTU and RTF vials depends on Your production scale, budget constraints, and willingness to invest in in-house sterilization capabilities.
Choosing the Right Vial Solution for Your Needs
Selecting the appropriate vial solution depends on production scale, sterility requirements, and cost-efficiency. Ready-to-use vials offer pre-sterilized convenience, reducing contamination risk and setup time, ideal for small batch productions or urgent pharmaceutical needs. Ready-to-fill vials provide customization and flexibility for large-scale manufacturing, enabling in-house sterilization and quality control tailored to specific formulations.
Ready-to-use Vial vs Ready-to-fill Vial Infographic
 libmatt.com
libmatt.com