Orphan drugs are developed to treat rare diseases affecting small patient populations, while blockbuster drugs target common conditions with large market potential, generating substantial revenue. Understanding the difference helps you appreciate the distinct regulatory incentives and market challenges each drug type faces.
Table of Comparison
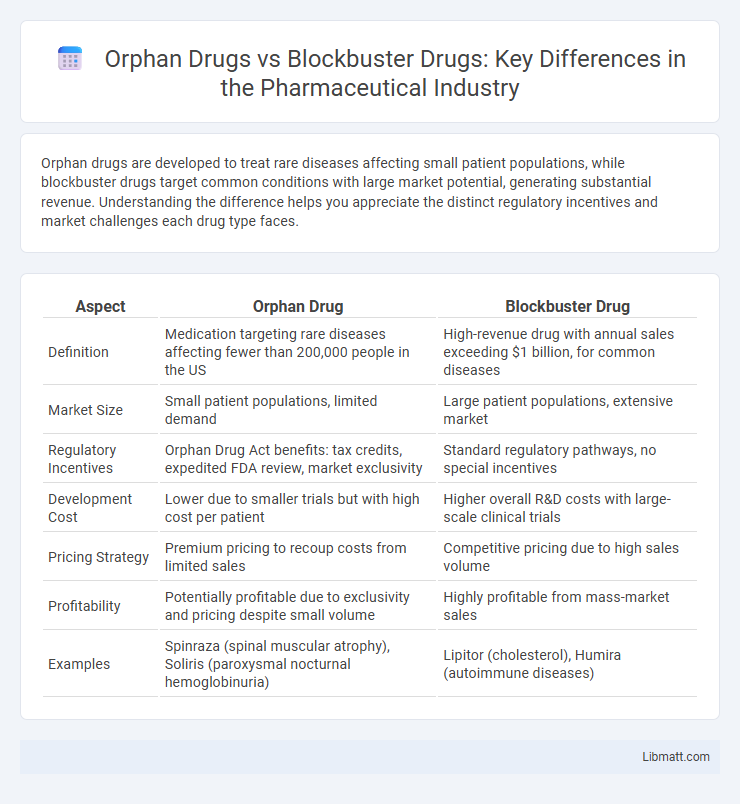
| Aspect | Orphan Drug | Blockbuster Drug |
|---|---|---|
| Definition | Medication targeting rare diseases affecting fewer than 200,000 people in the US | High-revenue drug with annual sales exceeding $1 billion, for common diseases |
| Market Size | Small patient populations, limited demand | Large patient populations, extensive market |
| Regulatory Incentives | Orphan Drug Act benefits: tax credits, expedited FDA review, market exclusivity | Standard regulatory pathways, no special incentives |
| Development Cost | Lower due to smaller trials but with high cost per patient | Higher overall R&D costs with large-scale clinical trials |
| Pricing Strategy | Premium pricing to recoup costs from limited sales | Competitive pricing due to high sales volume |
| Profitability | Potentially profitable due to exclusivity and pricing despite small volume | Highly profitable from mass-market sales |
| Examples | Spinraza (spinal muscular atrophy), Soliris (paroxysmal nocturnal hemoglobinuria) | Lipitor (cholesterol), Humira (autoimmune diseases) |
Introduction to Orphan Drugs and Blockbuster Drugs
Orphan drugs target rare diseases affecting fewer than 200,000 people in the U.S., often qualifying for incentives like market exclusivity and tax credits to encourage development. Blockbuster drugs generate annual sales exceeding $1 billion, typically treating common conditions with large patient populations. Understanding these distinctions is crucial for pharmaceutical strategies, regulatory pathways, and market dynamics.
Defining Orphan Drugs: Characteristics and Criteria
Orphan drugs are pharmaceutical agents developed specifically to treat rare diseases affecting fewer than 200,000 people in the United States or the equivalent rarity threshold in other regions, as defined by regulatory bodies like the FDA and EMA. These drugs often receive special status, including market exclusivity, tax credits, and research subsidies to incentivize development despite limited patient populations. Characteristic attributes include high development costs relative to small market size, specialized clinical trials, and a focus on addressing unmet medical needs in rare disease communities.
Understanding Blockbuster Drugs: Scale and Impact
Blockbuster drugs generate annual sales exceeding $1 billion, driving significant revenue and shaping pharmaceutical market trends through widespread use. These drugs target common chronic conditions such as diabetes, cardiovascular diseases, and depression, ensuring large patient populations and consistent demand. The scale of blockbuster drugs fuels substantial investment in marketing and innovation, reinforcing their dominant market presence.
Regulatory Frameworks for Orphan and Blockbuster Drugs
Regulatory frameworks for orphan drugs are designed to encourage development through incentives such as market exclusivity, tax credits, and expedited approval processes granted by agencies like the FDA and EMA. In contrast, blockbuster drugs face more standard regulatory pathways with greater scrutiny due to their large market impact and widespread use. Your ability to navigate these distinct frameworks can significantly affect drug development timelines and commercial success.
Market Dynamics: Niche vs. Mass Appeal
Orphan drugs target rare diseases affecting small patient populations, resulting in niche market dynamics with high per-patient costs and limited competition. Blockbuster drugs cater to widespread conditions, generating mass appeal and massive sales volumes, often exceeding billions annually. Your strategic focus should align with whether you aim to capture specialized markets with premium pricing or large markets driven by volume sales.
Research and Development Challenges
Orphan drugs face significant research and development challenges due to limited patient populations and scarce clinical trial data, increasing the complexity and cost of proving efficacy and safety. Blockbuster drugs benefit from larger target markets, allowing for extensive phase trials and substantial investment in advanced technologies to optimize drug formulations and delivery. The high financial risk in orphan drug R&D often necessitates regulatory incentives like market exclusivity and tax credits to encourage innovation in rare disease treatments.
Pricing Strategies and Economic Considerations
Orphan drugs typically have higher prices due to limited patient populations and significant R&D investments, supported by incentives like market exclusivity and tax credits. Blockbuster drugs rely on large-scale sales volumes to maximize revenue, enabling more moderate per-unit pricing and broader market access. Your choice between these drug types impacts pricing strategies, reimbursement policies, and long-term economic sustainability in healthcare markets.
Patient Access and Healthcare Outcomes
Orphan drugs target rare diseases affecting small patient populations, often resulting in limited but critical access for patients due to high costs and specialized distribution. Blockbuster drugs serve broad markets, improving healthcare outcomes on a larger scale by treating common conditions and ensuring widespread availability and affordability. Patient access to orphan drugs is frequently challenged by pricing and insurance coverage, whereas blockbuster drugs benefit from economies of scale and established healthcare pathways that facilitate treatment adherence and outcome monitoring.
Innovation and Future Trends in Drug Development
Orphan drugs drive innovation by targeting rare diseases with limited treatment options, promoting personalized medicine and novel therapeutic approaches. Blockbuster drugs, while focusing on widespread conditions, stimulate advancements in large-scale production and market-driven research. Future trends indicate a convergence of precision medicine and digital health technologies, fostering more efficient drug discovery and tailored treatments across both orphan and blockbuster drug development.
Orphan Drug vs. Blockbuster Drug: Key Takeaways and Comparisons
Orphan drugs target rare diseases affecting fewer than 200,000 people in the U.S., offering specialized treatment options with incentives like market exclusivity and tax credits to encourage development. Blockbuster drugs generate over $1 billion in annual revenue by addressing widespread conditions, often benefiting from large-scale marketing and broad patient populations. Understanding the differences in market size, development costs, and regulatory pathways can help you navigate pharmaceutical strategies and investment opportunities.
Orphan drug vs blockbuster drug Infographic
 libmatt.com
libmatt.com