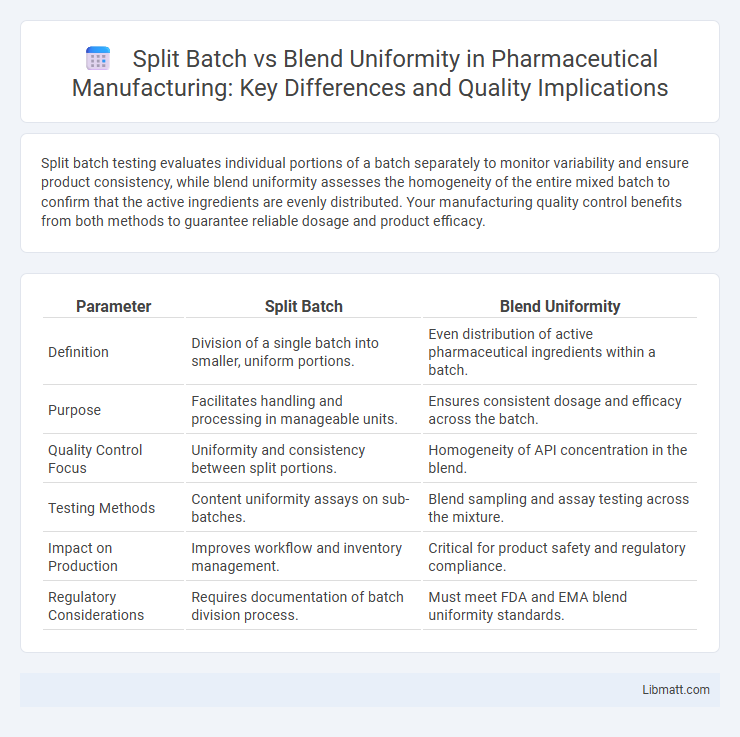
Split batch testing evaluates individual portions of a batch separately to monitor variability and ensure product consistency, while blend uniformity assesses the homogeneity of the entire mixed batch to confirm that the active ingredients are evenly distributed. Your manufacturing quality control benefits from both methods to guarantee reliable dosage and product efficacy.
Table of Comparison
| Parameter | Split Batch | Blend Uniformity |
|---|---|---|
| Definition | Division of a single batch into smaller, uniform portions. | Even distribution of active pharmaceutical ingredients within a batch. |
| Purpose | Facilitates handling and processing in manageable units. | Ensures consistent dosage and efficacy across the batch. |
| Quality Control Focus | Uniformity and consistency between split portions. | Homogeneity of API concentration in the blend. |
| Testing Methods | Content uniformity assays on sub-batches. | Blend sampling and assay testing across the mixture. |
| Impact on Production | Improves workflow and inventory management. | Critical for product safety and regulatory compliance. |
| Regulatory Considerations | Requires documentation of batch division process. | Must meet FDA and EMA blend uniformity standards. |
Introduction to Batch Splitting and Blend Uniformity
Batch splitting involves dividing a large production batch into smaller portions to improve process control and adaptability in pharmaceutical manufacturing. Blend uniformity measures the even distribution of active ingredients within a mixture, ensuring consistent dosage and efficacy across all units. Maintaining high blend uniformity during batch splitting is critical to prevent content variability and guarantee product quality.
Definitions: What is Split Batch?
Split batch refers to a manufacturing process where a single batch of material is divided into separate portions processed independently, which can lead to variations in quality and consistency. Blend uniformity measures how evenly components are distributed within a batch, ensuring consistent product performance. Understanding Split batch versus blend uniformity helps you optimize production methods to maintain product efficacy and compliance with quality standards.
Definitions: What is Blend Uniformity?
Blend uniformity refers to the consistent distribution of active pharmaceutical ingredients (APIs) throughout a powder mixture to ensure each dosage unit contains the intended amount of each component. It is critical in pharmaceutical manufacturing to guarantee product efficacy and safety by minimizing variability between doses. Split batch, in contrast, involves dividing a production batch into smaller portions, which can impact blend uniformity if mixing is not adequately maintained.
Regulatory Guidelines for Batch Splitting and Blend Uniformity
Regulatory guidelines such as FDA's 21 CFR Part 211 and ICH Q8 emphasize stringent control over split batch production and blend uniformity to ensure product quality and consistency. Batch splitting requires validation to confirm that each sub-batch maintains equivalent critical quality attributes, while blend uniformity testing ensures homogeneity of the active pharmaceutical ingredient (API) within a batch. Compliance with these regulations involves rigorous sampling, analytical testing, and documented evidence demonstrating consistent potency, uniformity, and safety across all split and blended batches.
Causes and Risks of Split Batch Processing
Split batch processing often causes variability due to inconsistent mixing and uneven distribution of ingredients. This inconsistency increases the risk of reduced product efficacy, quality control failures, and regulatory non-compliance. Ensuring blend uniformity is critical to maintain Your product's safety and performance standards during manufacturing.
Importance of Blend Uniformity in Pharmaceutical Manufacturing
Blend uniformity ensures consistent distribution of active pharmaceutical ingredients (APIs) throughout a batch, critical for guaranteeing dosage accuracy and therapeutic efficacy. Poor blend uniformity can lead to variations in drug potency, affecting product safety and regulatory compliance. Maintaining stringent blend uniformity standards supports quality control, reduces batch failures, and meets FDA and EMA guidelines in pharmaceutical manufacturing.
Methods for Testing Blend Uniformity
Methods for testing blend uniformity include sample collection at multiple locations within the batch to ensure representative analysis. Analytical techniques like near-infrared spectroscopy (NIR) and high-performance liquid chromatography (HPLC) provide precise quantification of component distribution, critical for both split batch and blend uniformity assessments. You can improve product consistency by applying validated sampling protocols and real-time monitoring technologies during manufacturing.
Impact of Split Batch on Final Product Quality
Split batch processing can lead to variability in blend uniformity, directly impacting the final product quality by causing inconsistencies in content uniformity and potency. Differences in mixing times or batch handling between split portions may result in uneven distribution of active pharmaceutical ingredients, risking dosage accuracy and product efficacy. Ensuring strict control of split batch processes is crucial to maintaining your product's uniformity and meeting regulatory quality standards.
Strategies to Improve Blend Uniformity
Implementing advanced mixing technologies such as high-shear mixers and continuous blending systems significantly enhances blend uniformity by ensuring consistent particle distribution. Optimizing process parameters, including mixing time, speed, and sequence of ingredient addition, reduces variability across batches and prevents segregation. Regular monitoring through near-infrared (NIR) spectroscopy and other non-destructive analytical techniques allows early detection of uniformity issues, enabling timely adjustments to maintain product quality.
Conclusion: Ensuring Consistency in Pharmaceutical Production
Split batch and blend uniformity are critical quality attributes that directly impact dosage accuracy and therapeutic efficacy in pharmaceutical production. Maintaining strict control over blend uniformity prevents content variability, while effective split batch execution ensures each subdivided portion retains the desired composition. Ensuring consistency through validated processes and real-time monitoring enhances product reliability and regulatory compliance.
Split batch vs Blend uniformity Infographic
 libmatt.com
libmatt.com