Nuclear Magnetic Resonance (NMR) provides detailed insights into the molecular structure and dynamics by analyzing the magnetic properties of atomic nuclei, while Mass Spectrometry (MS) excels in determining molecular mass and identifying compounds based on their mass-to-charge ratio. Your choice depends on whether structural information or precise molecular weight and composition data are more critical for your analysis.
Table of Comparison
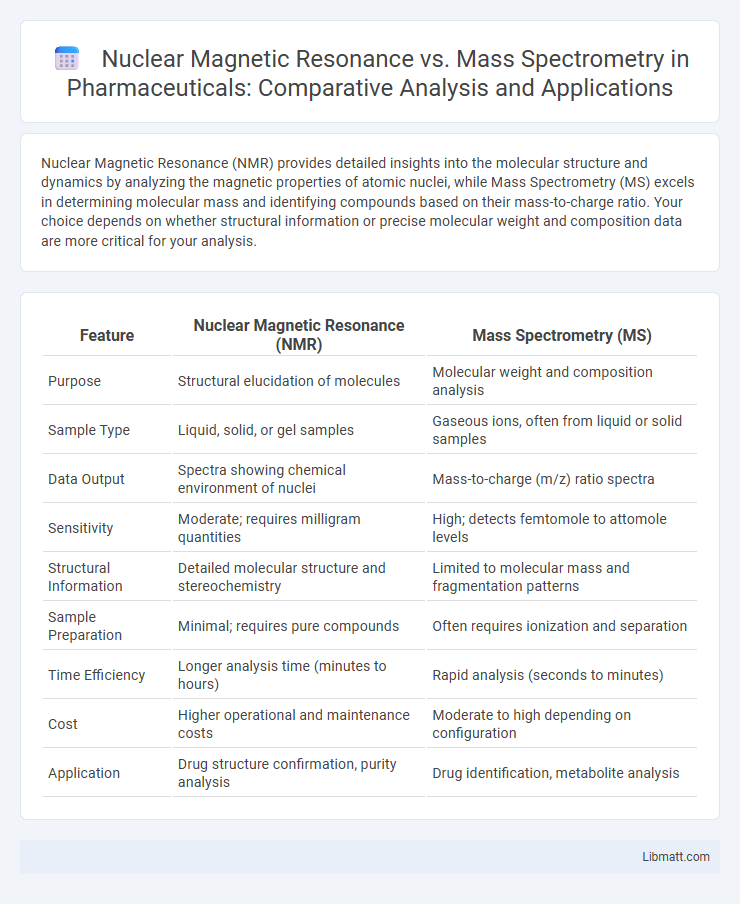
| Feature | Nuclear Magnetic Resonance (NMR) | Mass Spectrometry (MS) |
|---|---|---|
| Purpose | Structural elucidation of molecules | Molecular weight and composition analysis |
| Sample Type | Liquid, solid, or gel samples | Gaseous ions, often from liquid or solid samples |
| Data Output | Spectra showing chemical environment of nuclei | Mass-to-charge (m/z) ratio spectra |
| Sensitivity | Moderate; requires milligram quantities | High; detects femtomole to attomole levels |
| Structural Information | Detailed molecular structure and stereochemistry | Limited to molecular mass and fragmentation patterns |
| Sample Preparation | Minimal; requires pure compounds | Often requires ionization and separation |
| Time Efficiency | Longer analysis time (minutes to hours) | Rapid analysis (seconds to minutes) |
| Cost | Higher operational and maintenance costs | Moderate to high depending on configuration |
| Application | Drug structure confirmation, purity analysis | Drug identification, metabolite analysis |
Introduction to NMR and Mass Spectrometry
Nuclear Magnetic Resonance (NMR) spectroscopy analyzes the magnetic properties of atomic nuclei to provide detailed information about molecular structure and dynamics. Mass Spectrometry (MS) identifies and quantifies compounds by measuring the mass-to-charge ratio of ionized particles, offering insights into molecular weight and composition. Your choice between NMR and MS depends on the specific analytical goals, such as structural elucidation versus precise molecular mass determination.
Fundamental Principles of NMR
Nuclear Magnetic Resonance (NMR) operates based on the magnetic properties of atomic nuclei, detecting the interaction of nuclear spins with an external magnetic field and radiofrequency pulses. This technique measures the resonance frequencies of nuclei such as hydrogen-1 or carbon-13 to reveal molecular structure, chemical environment, and dynamics. Unlike Mass Spectrometry, which ionizes molecules to measure mass-to-charge ratios, NMR provides detailed information about atomic connectivity and spatial arrangement through chemical shifts and spin-spin coupling patterns.
Fundamental Principles of Mass Spectrometry
Mass Spectrometry operates on the principle of ionizing chemical compounds to generate charged molecules or fragments, which are then separated based on their mass-to-charge ratio using electromagnetic fields. The technique provides precise molecular weight and structural information by detecting the mass spectrum of ions with high sensitivity and accuracy. Your choice between Nuclear Magnetic Resonance and Mass Spectrometry depends on whether you require detailed molecular structure elucidation or exact molecular mass determination.
Sample Preparation Requirements
Nuclear Magnetic Resonance (NMR) requires minimal sample preparation, often needing samples to be dissolved in deuterated solvents to avoid interference from hydrogen atoms in the solvent. Mass Spectrometry (MS) demands more extensive preparation, including ionization methods such as electrospray or MALDI, and often requires samples to be purified and possibly derivatized for effective analysis. The complexity of MS sample preparation is critical for achieving accurate mass-to-charge ratio measurements and reducing matrix effects.
Data Output and Interpretation
Nuclear Magnetic Resonance (NMR) provides detailed spectral data representing the magnetic environment of atomic nuclei, allowing precise structural elucidation and quantification of molecular components. Mass Spectrometry (MS) generates mass-to-charge ratio spectra that depict molecular weights and fragmentation patterns, facilitating compound identification and molecular formula determination. Your choice between NMR and MS depends on whether you prioritize detailed structural information or high sensitivity in molecular mass analysis.
Sensitivity and Detection Limits
Nuclear Magnetic Resonance (NMR) typically exhibits lower sensitivity and higher detection limits compared to Mass Spectrometry (MS), often requiring millimolar concentrations for accurate analysis. MS offers superior sensitivity, detecting molecules at femtomolar to picomolar levels, making it ideal for trace detection in complex mixtures. Your choice between NMR and MS should consider the sensitivity needs of your sample to ensure precise and reliable characterization.
Applications in Chemical Analysis
Nuclear Magnetic Resonance (NMR) excels in elucidating detailed molecular structures and dynamics, making it invaluable for identifying organic compounds and studying chemical reactions. Mass Spectrometry (MS) offers precise molecular weight determination and sensitive detection of complex mixtures, essential for proteomics, metabolomics, and trace analysis. Your choice depends on whether structural information (NMR) or mass-based identification and quantification (MS) is the primary goal in chemical analysis.
Advantages and Limitations of NMR
Nuclear Magnetic Resonance (NMR) spectroscopy offers non-destructive detailed structural information, including molecular dynamics and stereochemistry, making it invaluable for analyzing complex organic compounds and biomolecules. Its primary limitations include lower sensitivity compared to Mass Spectrometry (MS), requiring larger sample quantities, and longer acquisition times, which can restrict high-throughput analysis. NMR also faces challenges with overlapping signals in complex mixtures, whereas MS excels in sensitivity and mass determination but provides less detailed structural information.
Advantages and Limitations of Mass Spectrometry
Mass spectrometry offers high sensitivity and specificity, enabling precise molecular weight determination and structural elucidation of complex compounds. Its rapid analysis and ability to handle mixtures without extensive separation make it invaluable in proteomics and metabolomics. Limitations include the necessity for ionizable samples, potential fragmentation complicating data interpretation, and high instrument costs and maintenance requirements.
Choosing Between NMR and Mass Spectrometry
Choosing between Nuclear Magnetic Resonance (NMR) and Mass Spectrometry (MS) depends on your analytical goals, as NMR provides detailed information about molecular structure, including stereochemistry and electronic environment. MS offers high sensitivity and precise molecular weight determination, making it ideal for identifying compounds in complex mixtures. You should select NMR for structural elucidation and MS for rapid identification and quantification in your experiments.
Nuclear Magnetic Resonance vs Mass Spectrometry Infographic
 libmatt.com
libmatt.com