Hydrolysis breaks chemical bonds through the addition of water, often splitting molecules into smaller components, while oxidation involves the loss of electrons and typically increases the oxygen content or decreases hydrogen in a substance. Understanding the difference between hydrolysis and oxidation is crucial for grasping many biological and chemical processes affecting Your reactions and products.
Table of Comparison
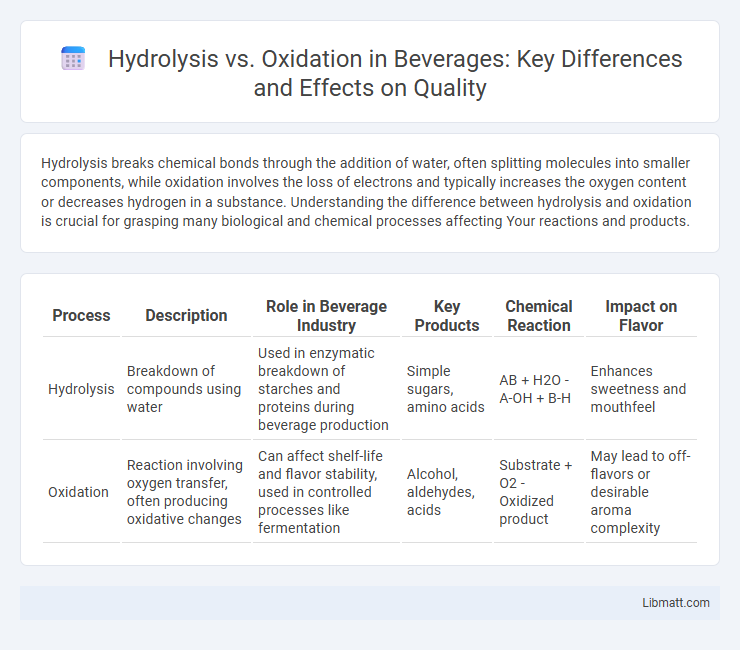
| Process | Description | Role in Beverage Industry | Key Products | Chemical Reaction | Impact on Flavor |
|---|---|---|---|---|---|
| Hydrolysis | Breakdown of compounds using water | Used in enzymatic breakdown of starches and proteins during beverage production | Simple sugars, amino acids | AB + H2O - A-OH + B-H | Enhances sweetness and mouthfeel |
| Oxidation | Reaction involving oxygen transfer, often producing oxidative changes | Can affect shelf-life and flavor stability, used in controlled processes like fermentation | Alcohol, aldehydes, acids | Substrate + O2 - Oxidized product | May lead to off-flavors or desirable aroma complexity |
Introduction to Hydrolysis and Oxidation
Hydrolysis is a chemical reaction involving the breaking of a bond in a molecule using water, commonly seen in the digestion of food or the breakdown of polymers. Oxidation refers to the loss of electrons from a molecule, atom, or ion, playing a crucial role in processes like cellular respiration and combustion. Understanding the differences between hydrolysis and oxidation helps you grasp essential biochemical and industrial reactions.
Defining Hydrolysis: Key Concepts
Hydrolysis is a chemical reaction involving the breaking of bonds in molecules through the addition of water, typically splitting compounds into smaller units. Unlike oxidation, which involves the loss of electrons and often reacts with oxygen, hydrolysis specifically targets the cleavage of bonds such as esters, peptides, or glycosidic linkages. Understanding hydrolysis is crucial for Your grasp of biochemical processes, industrial applications, and environmental chemistry where water-mediated reactions predominate.
Defining Oxidation: Essential Principles
Oxidation involves the loss of electrons or an increase in oxidation state of a molecule, atom, or ion, often by reaction with oxygen or other oxidizing agents. It plays a key role in various chemical and biological processes, including cellular respiration and combustion. Understanding the essential principles of oxidation helps you distinguish it clearly from hydrolysis, which primarily involves the cleavage of bonds through the addition of water.
Chemical Mechanisms: How Hydrolysis Works
Hydrolysis involves the cleavage of chemical bonds through the addition of water, typically breaking esters, amides, or glycosidic bonds into smaller molecules. The reaction proceeds via nucleophilic attack of water or hydroxide ions on the electrophilic carbonyl carbon, leading to bond fragmentation and formation of hydroxyl and other functional groups. This mechanism contrasts with oxidation, where electron transfer alters oxidation states rather than bond cleavage by water molecules.
Chemical Mechanisms: How Oxidation Occurs
Oxidation occurs through the loss of electrons from a molecule, atom, or ion, often involving the addition of oxygen or the removal of hydrogen. This chemical mechanism transforms the oxidation state of the substance, facilitating reactions such as combustion, respiration, and corrosion. Understanding this process helps you distinguish it from hydrolysis, which involves breaking chemical bonds with water rather than electron transfer.
Major Differences Between Hydrolysis and Oxidation
Hydrolysis involves the breaking of chemical bonds through the addition of water, typically splitting molecules into smaller units, while oxidation refers to the loss of electrons from a molecule, often involving oxygen and resulting in an increase in oxidation state. Hydrolysis is commonly seen in biological processes such as digestion, where complex molecules like proteins and carbohydrates are broken down, whereas oxidation plays a crucial role in energy production and metabolic pathways like cellular respiration. Understanding these major differences helps you distinguish between the mechanisms and outcomes of these fundamental chemical reactions.
Real-World Examples of Hydrolysis
Hydrolysis plays a crucial role in digesting carbohydrates, proteins, and lipids, breaking down complex molecules into absorbable units like glucose, amino acids, and fatty acids. In industrial applications, hydrolysis is essential for producing biofuels by converting biomass into fermentable sugars. Enzymatic hydrolysis also enables wastewater treatment by decomposing organic pollutants, enhancing environmental sustainability.
Real-World Applications of Oxidation
Oxidation plays a critical role in real-world applications such as energy production, where it drives combustion in engines and power plants to generate electricity. It is essential in environmental processes like the degradation of pollutants and in industrial manufacturing for rust prevention through controlled oxidation. Your understanding of oxidation can improve technologies in waste treatment and material science, enhancing sustainability efforts globally.
Importance in Biology and Industry
Hydrolysis plays a crucial role in biology by facilitating the breakdown of complex molecules like proteins, carbohydrates, and lipids into simpler units essential for cellular functions and energy release. Oxidation is vital in cellular respiration, enabling cells to convert nutrients into usable energy through electron transfer processes, and is extensively used in industries for energy production and waste treatment. Your understanding of these reactions supports innovations in biotechnology and sustainable industrial processes.
Comparing Impact: Hydrolysis vs Oxidation
Hydrolysis breaks chemical bonds through the reaction with water, significantly altering molecular structures, especially in organic compounds and polymers, resulting in decomposition or transformation. Oxidation involves the transfer of electrons, often introducing oxygen or removing hydrogen, leading to changes in chemical composition and energy release, critically impacting metabolic and industrial processes. Understanding the comparative impact of hydrolysis versus oxidation helps you determine which reaction mechanism influences product stability, degradation rates, or energy conversion efficiency in specific chemical or biological systems.
Hydrolysis vs oxidation Infographic
 libmatt.com
libmatt.com