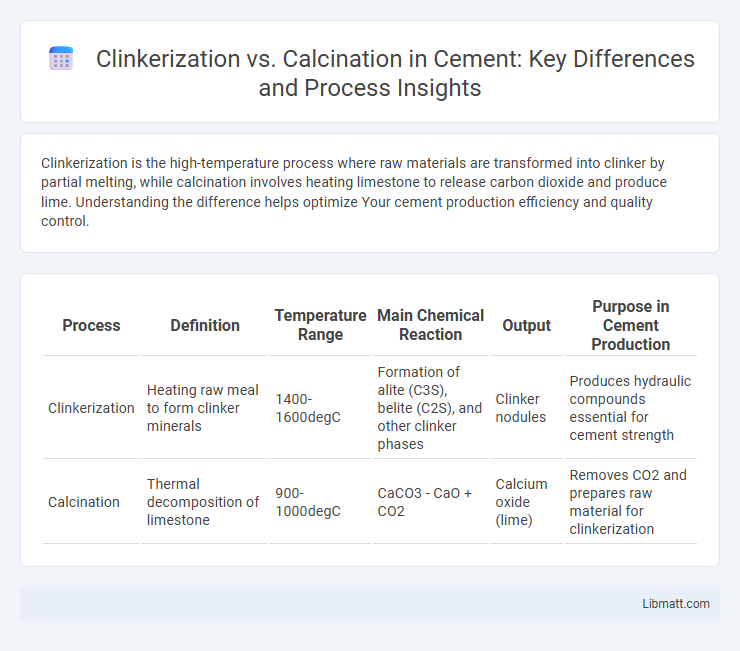
Clinkerization is the high-temperature process where raw materials are transformed into clinker by partial melting, while calcination involves heating limestone to release carbon dioxide and produce lime. Understanding the difference helps optimize Your cement production efficiency and quality control.
Table of Comparison
| Process | Definition | Temperature Range | Main Chemical Reaction | Output | Purpose in Cement Production |
|---|---|---|---|---|---|
| Clinkerization | Heating raw meal to form clinker minerals | 1400-1600degC | Formation of alite (C3S), belite (C2S), and other clinker phases | Clinker nodules | Produces hydraulic compounds essential for cement strength |
| Calcination | Thermal decomposition of limestone | 900-1000degC | CaCO3 - CaO + CO2 | Calcium oxide (lime) | Removes CO2 and prepares raw material for clinkerization |
Introduction to Clinkerization and Calcination
Clinkerization is a high-temperature process in cement manufacturing where raw meal is transformed into clinker by partial melting in a rotary kiln at temperatures around 1450degC. Calcination involves the thermal decomposition of limestone (CaCO3) into lime (CaO) and carbon dioxide (CO2) at approximately 900degC, serving as a critical step that precedes clinkerization. Both processes are essential for producing cement, with calcination initiating chemical changes and clinkerization completing mineral formation.
Defining Clinkerization: Key Processes
Clinkerization involves heating a mixture of raw materials, primarily limestone and clay, in a rotary kiln to form clinker nodules, essential for cement production. Key processes include chemical reactions like decarbonation, sintering, and the formation of calcium silicates, which determine the final clinker quality. Understanding clinkerization ensures Your cement manufacturing achieves optimal strength and durability compared to calcination, which focuses solely on thermal decomposition without clinker formation.
Understanding Calcination: Core Principles
Calcination is a thermal treatment process that drives off volatile substances or induces phase changes in materials like limestone, essential for cement manufacturing. Unlike clinkerization, which involves the formation of clinker at high temperatures around 1450degC, calcination occurs at lower temperatures typically between 700-1000degC to convert calcium carbonate into calcium oxide and carbon dioxide. This endothermic reaction sets the foundation for subsequent clinker formation, making calcination a critical step in cement production efficiency and quality control.
Raw Materials Involved in Clinkerization and Calcination
Clinkerization involves the raw materials limestone, clay, and shale, which are heated in a kiln to form clinker--a nodular material essential for cement production. Calcination primarily involves limestone (calcium carbonate), which is thermally decomposed into lime (calcium oxide) and carbon dioxide at around 900degC. The distinct raw material focus differentiates clinkerization's complex mixture from calcination's single primary input.
Chemical Reactions and Thermal Processes
Clinkerization involves the chemical transformation of raw materials like limestone and clay into clinker by heating them in a kiln at temperatures around 1450degC, causing reactions such as decarbonation of calcium carbonate and formation of calcium silicates. Calcination primarily refers to the thermal decomposition of calcium carbonate into calcium oxide and carbon dioxide at lower temperatures, typically around 900degC, without forming clinker phases. The clinkerization process couples complex phase changes with sintering reactions, while calcination is largely a single-step thermal decomposition reaction.
Equipment Used in Clinkerization vs Calcination
Clinkerization primarily involves the use of rotary kilns operating at high temperatures around 1400-1500degC to facilitate the chemical transformation of raw meal into clinker nodules. Calcination utilizes rotary calciners or shaft calciners to preheat and partially decompose raw materials at lower temperatures approximately 850-1000degC before clinkerization. The advanced design of rotary kilns ensures efficient heat transfer and thorough mixing, while calciners focus on optimizing fuel consumption and reducing emission during the decarbonation phase.
Energy Requirements and Efficiency Comparison
Clinkerization demands significantly higher energy inputs than calcination due to the elevated temperatures reaching around 1450degC required for clinker formation, compared to approximately 900degC for calcination. Energy efficiency in clinkerization is typically lower because the process involves complex chemical reactions and phase changes, leading to greater fuel consumption and heat loss. Your choice between these processes can impact operational costs and environmental footprints, with calcination offering a more energy-efficient option in cement production phases focused on dehydration and chemical transformation of raw materials.
Environmental Impact: Emissions and Byproducts
Clinkerization releases significant CO2 and other pollutants due to the high-temperature reaction of limestone with clay, making it a major contributor to greenhouse gas emissions in cement production. Calcination, while also involving the thermal decomposition of carbonate minerals, typically produces fewer emissions as it occurs at lower temperatures and is often associated with alternative industrial processes. Understanding these differences helps you evaluate cleaner manufacturing options and reduces the overall environmental footprint of cement production.
Applications in Industrial Sectors
Clinkerization is primarily employed in the cement manufacturing industry, where it transforms raw materials into clinker through high-temperature processing, essential for producing hydraulic cement. Calcination finds extensive use in various industrial sectors such as metallurgy, chemical manufacturing, and building materials, where it decomposes carbonates, hydroxides, or hydrates by heating to create oxides and release volatile components. Your choice between clinkerization and calcination processes depends on the specific industrial application, such as cement production for construction versus metal oxide preparation in chemical industries.
Future Trends and Technological Advancements
Future trends in clinkerization emphasize energy efficiency and carbon capture technologies, aiming to reduce CO2 emissions in cement production. Innovations in alternative fuel utilization and electrification of kiln processes are driving technological advancements in calcination, enhancing sustainability. Integration of AI and real-time monitoring systems is optimizing operational performance for both clinkerization and calcination stages.
Clinkerization vs Calcination Infographic
 libmatt.com
libmatt.com