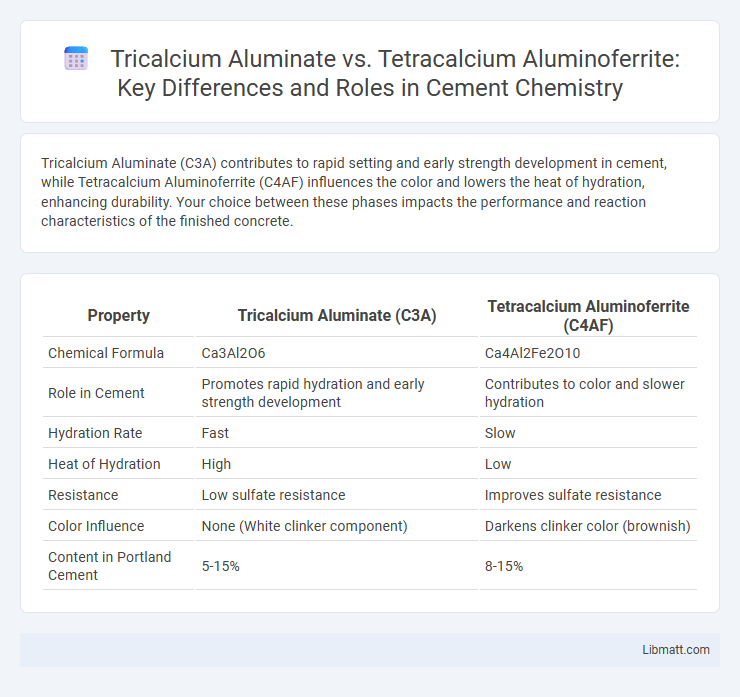
Tricalcium Aluminate (C3A) contributes to rapid setting and early strength development in cement, while Tetracalcium Aluminoferrite (C4AF) influences the color and lowers the heat of hydration, enhancing durability. Your choice between these phases impacts the performance and reaction characteristics of the finished concrete.
Table of Comparison
| Property | Tricalcium Aluminate (C3A) | Tetracalcium Aluminoferrite (C4AF) |
|---|---|---|
| Chemical Formula | Ca3Al2O6 | Ca4Al2Fe2O10 |
| Role in Cement | Promotes rapid hydration and early strength development | Contributes to color and slower hydration |
| Hydration Rate | Fast | Slow |
| Heat of Hydration | High | Low |
| Resistance | Low sulfate resistance | Improves sulfate resistance |
| Color Influence | None (White clinker component) | Darkens clinker color (brownish) |
| Content in Portland Cement | 5-15% | 8-15% |
Introduction to Tricalcium Aluminate and Tetracalcium Aluminoferrite
Tricalcium aluminate (C3A) is a key phase in Portland cement clinker known for its rapid hydration and contribution to early strength development. Tetracalcium aluminoferrite (C4AF) is another clinker phase that hydrates slower and imparts a distinct color and durability properties to cement. Both compounds significantly influence the chemical and physical characteristics of cement, affecting setting time, heat generation, and overall performance in concrete applications.
Chemical Structures and Formulas
Tricalcium aluminate (C3A) has the chemical formula Ca3Al2O6 and crystallizes in a cubic structure, characterized by isolated AlO4 tetrahedra linked by calcium ions. Tetracalcium aluminoferrite (C4AF) is represented by the formula Ca4Al2Fe2O10, featuring a complex orthorhombic lattice where aluminum and iron ions substitute within octahedral sites. The substitution of Fe3+ in C4AF alters its magnetic and refractory properties compared to the purely aluminate phase in C3A.
Roles in Cement Chemistry
Tricalcium Aluminate (C3A) plays a crucial role in cement by influencing the setting time and early strength development, rapidly reacting with water and gypsum to control the initial hydration process. Tetracalcium Aluminoferrite (C4AF) contributes primarily to the color and overall durability of cement, reacting more slowly and providing resistance to chemical attack and sulfate environments. These compounds together balance the cement's hydration kinetics, mechanical properties, and resistance to environmental factors.
Physical and Mechanical Properties
Tricalcium Aluminate (C3A) exhibits high hydraulic reactivity and rapid setting times, contributing to early strength development in cementitious materials, while Tetracalcium Aluminoferrite (C4AF) offers moderate strength gains and improved resistance to sulfate attack. C3A's crystalline structure results in lower durability under aggressive chemical environments compared to C4AF, which contains iron, enhancing its stability and mechanical robustness. Understanding the distinct physical and mechanical properties of these phases can optimize Your cement formulations for targeted performance requirements.
Hydration Reactions and Products
Tricalcium Aluminate (C3A) exhibits rapid hydration, producing calcium aluminate hydrate and releasing heat that accelerates early cement setting, while Tetracalcium Aluminoferrite (C4AF) hydrates more slowly, forming hydrated ferrite phases with less heat evolution. The hydration of C3A results in ettringite formation in the presence of sulfate ions, important for concrete durability, whereas C4AF contributes to color and long-term strength through the formation of mixed calcium alumino-ferrite hydrates. Your understanding of these differences enhances control over cement setting times and overall concrete performance during construction.
Influence on Setting Time
Tricalcium Aluminate (C3A) significantly accelerates the initial setting time of cement due to its rapid hydration and high reactivity with water, causing faster early hardness development. In contrast, Tetracalcium Aluminoferrite (C4AF) has a minimal influence on setting time, primarily contributing to the cement's color and reducing clinker's energy consumption during manufacturing. Your choice between these compounds affects how quickly the cement will set, which is critical for scheduling construction processes and ensuring structural integrity.
Impact on Concrete Strength and Durability
Tricalcium Aluminate (C3A) significantly influences early concrete strength due to its rapid hydration, but it can reduce durability by increasing susceptibility to sulfate attack. Tetracalcium Aluminoferrite (C4AF) hydrates more slowly, contributing less to early strength but enhancing overall durability by improving resistance to chemical corrosion and reducing heat generation. Your concrete mix's balance of C3A and C4AF content is crucial for optimizing both strength and long-term durability.
Resistance to Sulfate Attack
Tricalcium Aluminate (C3A) exhibits low resistance to sulfate attack due to its high reactivity with sulfate ions, leading to the formation of expansive products like ettringite that cause concrete deterioration. In contrast, Tetracalcium Aluminoferrite (C4AF) has better sulfate resistance because it reacts less aggressively with sulfates and forms more stable phases under sulfate exposure. Concrete mixtures with reduced C3A content and higher proportions of C4AF improve durability in sulfate-rich environments, making C4AF preferred for sulfate-resistant cement formulations.
Industrial Applications and Significance
Tricalcium aluminate (C3A) plays a critical role in the early strength development and setting times of Portland cement, making it essential in rapid-hardening concrete and refractory applications due to its high reactivity with water. Tetracalcium aluminoferrite (C4AF) contributes to the color and durability of cement, providing resistance to sulfate attack and enhancing long-term strength in construction materials. Industrially, C3A influences hydration kinetics and heat release, while C4AF improves cost-effectiveness and performance in diverse environments, highlighting their complementary significance in cement manufacturing and concrete technology.
Comparative Summary and Key Differences
Tricalcium Aluminate (C3A) primarily influences early strength development and heat generation in cement hydration, while Tetracalcium Aluminoferrite (C4AF) contributes to the color of cement and enhances resistance to sulfate attacks. C3A reacts quickly with water, producing significant heat and enabling rapid setting times, whereas C4AF hydrates more slowly and provides improved durability under aggressive environmental conditions. Your choice between C3A and C4AF content impacts cement performance, balancing early strength needs with long-term chemical resistance.
Tricalcium Aluminate vs Tetracalcium Aluminoferrite Infographic
 libmatt.com
libmatt.com