Heat of hydration refers to the energy released when ions from a solute interact with water molecules during the formation of an aqueous solution, while heat of dissolution encompasses the total energy change when a solute dissolves, including both lattice energy and hydration energy. Understanding the difference helps you predict whether a dissolution process will be endothermic or exothermic based on the balance of these heat exchanges.
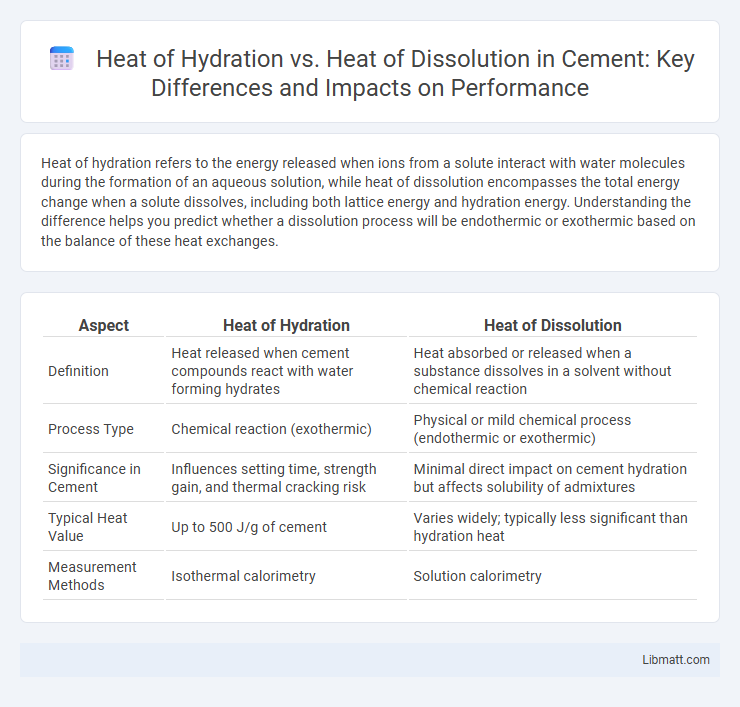
Table of Comparison
| Aspect | Heat of Hydration | Heat of Dissolution |
|---|---|---|
| Definition | Heat released when cement compounds react with water forming hydrates | Heat absorbed or released when a substance dissolves in a solvent without chemical reaction |
| Process Type | Chemical reaction (exothermic) | Physical or mild chemical process (endothermic or exothermic) |
| Significance in Cement | Influences setting time, strength gain, and thermal cracking risk | Minimal direct impact on cement hydration but affects solubility of admixtures |
| Typical Heat Value | Up to 500 J/g of cement | Varies widely; typically less significant than hydration heat |
| Measurement Methods | Isothermal calorimetry | Solution calorimetry |
Introduction to Heat of Hydration and Heat of Dissolution
Heat of hydration refers to the energy released when water molecules interact and form bonds with dissolved ions, typically observed during the solvation of ionic compounds. Heat of dissolution encompasses the total enthalpy change when a solute dissolves in a solvent, including both lattice energy disruption and hydration processes. Understanding these thermodynamic properties is essential for predicting solubility behavior and optimizing industrial separation or chemical synthesis processes.
Fundamental Thermodynamic Concepts
Heat of hydration refers to the energy released when water molecules surround and interact with ions during solvation, significantly influencing solution stability and enthalpy changes. Heat of dissolution encompasses the total enthalpy change when a solute dissolves, involving both lattice energy breakdown and hydration or solvation processes. The fundamental thermodynamic concept lies in the balance between endothermic lattice energy absorption and exothermic hydration energy release, determining whether the dissolution process is overall endothermic or exothermic.
Definition and Mechanism of Heat of Hydration
Heat of hydration refers to the energy released when water molecules surround and interact with ions or molecules during the dissolution process, forming a hydration shell through ion-dipole interactions. This exothermic process stabilizes the dissolved species by lowering the overall energy, driven primarily by the electrostatic attraction between water and solute particles. The heat of hydration is a critical factor in determining solubility and the thermodynamic properties of aqueous solutions.
Definition and Mechanism of Heat of Dissolution
Heat of dissolution refers to the amount of heat absorbed or released when a substance dissolves in a solvent, resulting from the breaking of solute-solute bonds and the formation of solute-solvent interactions. This process involves endothermic energy consumption to separate solute particles and exothermic energy release when solvation shells form around solute ions or molecules. Understanding the heat of dissolution is crucial for applications in chemistry and materials science, where solubility and temperature changes affect reaction dynamics.
Key Differences Between Hydration and Dissolution Processes
Heat of hydration refers to the energy released when water molecules surround and interact with ions or molecules during the formation of a hydrated compound, while heat of dissolution measures the total energy change when a solute dissolves in a solvent. Key differences include that hydration specifically involves water and ion interactions, producing enthalpy changes mostly from ion-dipole forces, whereas dissolution encompasses a broader range of solute-solvent interactions and may involve breaking and forming various types of bonds. Understanding these differences helps you accurately predict thermal effects in chemical processes involving solutes and solvents.
Factors Affecting Heat of Hydration
Heat of hydration is influenced by factors such as the ionic size, charge density, and lattice energy of the solute, which determine the strength of ion-water interactions. Temperature and the nature of the solvent, including dielectric constant and polarity, significantly affect the extent of hydration energy released. The degree of hydration also depends on the structural arrangement and hydration shell formed around ions during the process.
Factors Influencing Heat of Dissolution
Heat of dissolution is influenced by factors such as temperature, nature of solute and solvent, and the degree of solute-solvent interaction. Ionic compounds with strong lattice energy typically exhibit higher enthalpy changes due to the energy required to break ionic bonds. Solvent polarity and solute solubility directly affect the heat absorbed or released during the dissolution process.
Measurement Techniques for Enthalpy Changes
Measurement techniques for heat of hydration typically involve calorimetry methods such as isothermal calorimetry or conduction calorimetry, which precisely monitor temperature changes as water reacts with cementitious materials. Heat of dissolution is commonly measured using solution calorimetry, where the enthalpy change is recorded as a solute dissolves in a solvent under controlled temperature conditions. Differential scanning calorimetry (DSC) and titration calorimetry also provide accurate data on enthalpy changes for both hydration and dissolution processes by quantifying heat flow associated with chemical reactions.
Practical Applications in Industry and Research
Heat of Hydration and Heat of Dissolution play critical roles in industries such as pharmaceuticals, cement production, and chemical manufacturing, influencing product stability, reaction control, and energy management. Understanding the heat released or absorbed during these processes allows engineers and researchers to optimize formulation, enhance safety protocols, and improve process efficiency. Your ability to measure and manipulate these thermal effects directly impacts product quality and innovation in research and industrial applications.
Summary and Comparative Analysis
Heat of hydration refers to the energy released when water molecules surround and interact with ions in an ionic compound, while heat of dissolution is the overall energy change during the process of a solute dissolving in a solvent, including both lattice enthalpy and hydration energy. Typically, heat of hydration is exothermic, driven by ion-dipole interactions, whereas heat of dissolution can be endothermic or exothermic depending on the balance between lattice energy and hydration energy. Comparative analysis indicates that substances with high lattice enthalpy often require greater hydration energy to dissolve, influencing solubility and thermal behavior in aqueous solutions.
Heat of Hydration vs Heat of Dissolution Infographic
 libmatt.com
libmatt.com