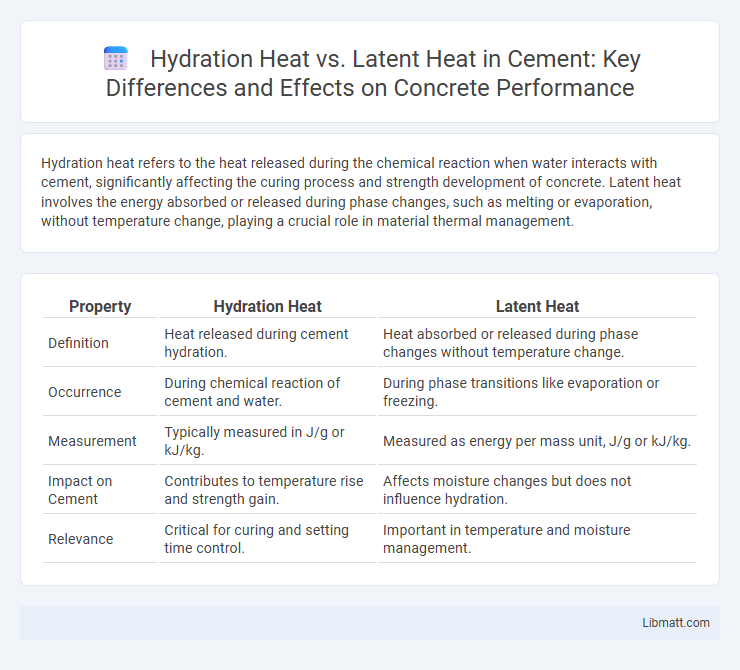
Hydration heat refers to the heat released during the chemical reaction when water interacts with cement, significantly affecting the curing process and strength development of concrete. Latent heat involves the energy absorbed or released during phase changes, such as melting or evaporation, without temperature change, playing a crucial role in material thermal management.
Table of Comparison
| Property | Hydration Heat | Latent Heat |
|---|---|---|
| Definition | Heat released during cement hydration. | Heat absorbed or released during phase changes without temperature change. |
| Occurrence | During chemical reaction of cement and water. | During phase transitions like evaporation or freezing. |
| Measurement | Typically measured in J/g or kJ/kg. | Measured as energy per mass unit, J/g or kJ/kg. |
| Impact on Cement | Contributes to temperature rise and strength gain. | Affects moisture changes but does not influence hydration. |
| Relevance | Critical for curing and setting time control. | Important in temperature and moisture management. |
Understanding Hydration Heat and Latent Heat
Hydration heat refers to the thermal energy released during the chemical reaction between cement and water, known as the hydration process, which significantly influences concrete setting and strength development. Latent heat is the energy absorbed or released during phase changes, such as melting or evaporation, without temperature change, playing a critical role in moisture and temperature regulation in construction materials. Understanding the distinction between hydration heat and latent heat is essential for optimizing curing conditions and improving the durability of concrete structures.
Key Differences Between Hydration Heat and Latent Heat
Hydration heat refers to the thermal energy released during a chemical reaction between cement and water, commonly observed in concrete curing processes, whereas latent heat is the energy absorbed or released during a phase change of a substance without temperature change, such as melting or condensation. Hydration heat is exothermic and influences setting time and strength development in cementitious materials, while latent heat governs phase transitions like ice melting or water vaporization. The key difference lies in hydration heat involving chemical reaction energy changes, whereas latent heat involves physical state transformations without altering chemical composition.
The Science Behind Hydration Heat
Hydration heat originates from the exothermic chemical reactions during the curing of cement when water molecules break down cement compounds like tricalcium silicate, releasing significant thermal energy that accelerates the hardening process. This energy release contrasts with latent heat, which involves the energy absorbed or released during phase changes, such as water evaporating or freezing, without changing temperature. Understanding hydration heat is critical for managing concrete curing conditions to prevent thermal cracking and ensure structural integrity in construction projects.
Exploring the Concept of Latent Heat
Latent heat refers to the amount of heat absorbed or released by a substance during a phase change, such as melting or vaporization, without changing its temperature. This energy is crucial in various physical and chemical processes where molecular bonds are broken or formed, distinguishing it from hydration heat, which involves heat exchange during the interaction of water with ions or molecules. Understanding latent heat is essential for fields like meteorology, material science, and chemical engineering, as it governs phase transitions and energy transfer efficiency.
Factors Influencing Hydration Heat
Hydration heat is influenced by factors such as cement composition, fineness, temperature, water-to-cement ratio, and curing conditions, which affect the rate of chemical reactions during cement hydration. Higher cement fineness and increased temperature accelerate hydration heat release, while lower water-to-cement ratios generally increase the heat generated per unit volume. The presence of supplementary cementitious materials like fly ash or slag can modify hydration heat by slowing down reaction rates and reducing overall heat evolution.
Practical Examples of Latent Heat
Latent heat plays a crucial role in practical applications such as ice melting, steam condensing in power plants, and refrigeration cycles where energy transfers occur without temperature changes. In your everyday life, latent heat is experienced when sweat evaporates from your skin, cooling your body through the phase change of water from liquid to vapor. Unlike hydration heat, which involves chemical reactions like cement setting, latent heat is primarily concerned with phase transitions and energy absorption or release during those processes.
Applications of Hydration Heat in Industry
Hydration heat plays a critical role in the construction industry, particularly in the curing of concrete where exothermic reactions influence strength development and setting time. Your understanding of hydration heat is essential for optimizing concrete mix designs to prevent thermal cracking in large structures like dams and bridges. This controlled heat release also benefits the manufacturing of refractory materials, ensuring durability and stability under high-temperature conditions.
Role of Latent Heat in Phase Changes
Latent heat plays a crucial role in phase changes by enabling the transformation between solid, liquid, and gas states without altering temperature. During phase transitions, such as melting or vaporization, latent heat energy is absorbed or released to break or form intermolecular bonds, facilitating changes in molecular arrangement. This energy exchange is essential for processes like water evaporation and ice melting, where heat drives the change of state independently of temperature variations.
Measuring Hydration Heat and Latent Heat
Measuring hydration heat involves calorimetric techniques such as isothermal calorimetry, which tracks the heat released during cement hydration reactions over time, providing insights into reaction kinetics and setting behavior. Latent heat is quantified using differential scanning calorimetry (DSC) to detect phase changes by recording heat absorbed or released, crucial for understanding material thermal properties during transitions like melting or crystallization. Accurate measurement of both hydration heat and latent heat enables optimization of thermal management in construction materials and your concrete mix design for enhanced durability and performance.
Importance of Heat Management in Engineering Processes
Effective heat management is crucial in engineering processes to control hydration heat, which is the exothermic reaction heat released during cement hydration, and latent heat, the energy absorbed or released during phase changes without temperature variation. Proper regulation of hydration heat prevents thermal cracking and ensures structural integrity in concrete construction, while managing latent heat optimizes temperature control in phase-change materials used in energy storage and thermal regulation systems. Understanding the distinct thermal properties and impacts of hydration heat and latent heat enables engineers to design systems with enhanced durability, efficiency, and safety.
Hydration Heat vs Latent Heat Infographic
 libmatt.com
libmatt.com