Amine scrubbing utilizes chemical absorption to selectively remove acidic gases like CO2 and H2S from gas streams, making it highly effective for gas purification in natural gas processing. Physical scrubbing relies on solvent solubility differences to separate gases without chemical reactions, offering faster regeneration and lower energy consumption for high-pressure gas streams but less selectivity compared to amine scrubbing.
Table of Comparison
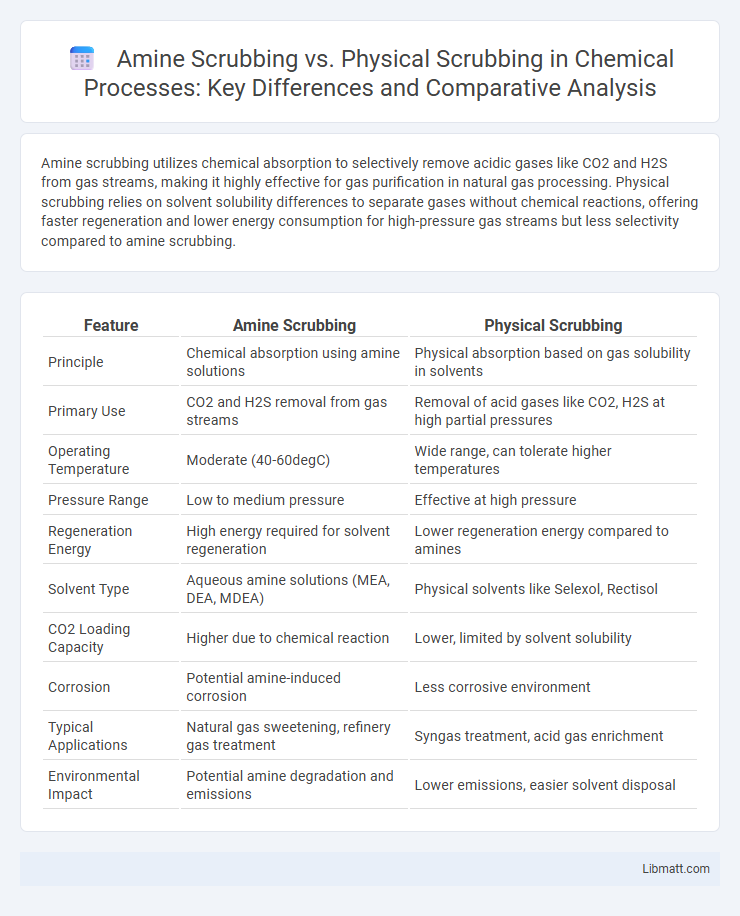
| Feature | Amine Scrubbing | Physical Scrubbing |
|---|---|---|
| Principle | Chemical absorption using amine solutions | Physical absorption based on gas solubility in solvents |
| Primary Use | CO2 and H2S removal from gas streams | Removal of acid gases like CO2, H2S at high partial pressures |
| Operating Temperature | Moderate (40-60degC) | Wide range, can tolerate higher temperatures |
| Pressure Range | Low to medium pressure | Effective at high pressure |
| Regeneration Energy | High energy required for solvent regeneration | Lower regeneration energy compared to amines |
| Solvent Type | Aqueous amine solutions (MEA, DEA, MDEA) | Physical solvents like Selexol, Rectisol |
| CO2 Loading Capacity | Higher due to chemical reaction | Lower, limited by solvent solubility |
| Corrosion | Potential amine-induced corrosion | Less corrosive environment |
| Typical Applications | Natural gas sweetening, refinery gas treatment | Syngas treatment, acid gas enrichment |
| Environmental Impact | Potential amine degradation and emissions | Lower emissions, easier solvent disposal |
Introduction to Gas Scrubbing Technologies
Gas scrubbing technologies, including amine scrubbing and physical scrubbing, are essential for removing contaminants such as CO2, H2S, and other acid gases from industrial gas streams. Amine scrubbing relies on chemical absorption using aqueous amine solutions, offering high selectivity and efficiency for low partial pressure gases, while physical scrubbing uses solvents like Selexol or Rectisol to absorb gases based on physical solubility, making it effective for high-pressure applications. Your choice between these technologies depends on the gas composition, pressure, and desired purity levels in the treated gas.
Overview of Amine Scrubbing
Amine scrubbing is a chemical absorption process primarily used for removing carbon dioxide (CO2) and hydrogen sulfide (H2S) from gas streams by reacting with amine solvents such as monoethanolamine (MEA) or methyldiethanolamine (MDEA). You benefit from its high selectivity and efficiency for acidic gas removal, making it ideal for natural gas sweetening and post-combustion CO2 capture in power plants. This process contrasts with physical scrubbing, which relies on gas solubility in solvents without chemical reaction, making amine scrubbing more effective for low partial pressure CO2 capture.
Overview of Physical Scrubbing
Physical scrubbing utilizes solvents such as Selexol, Rectisol, or methanol to absorb acidic gases like CO2 and H2S from gas streams based on their solubility differences, operating efficiently under high pressure. It involves reversible physical absorption without chemical reactions, making it especially effective for gas streams with high partial pressures of contaminants. This process offers advantages including lower energy consumption for solvent regeneration and faster absorption-desorption cycles compared to amine scrubbing.
Chemical Mechanisms: Amine vs Physical Processes
Amine scrubbing relies on chemical absorption where amine molecules react with acidic gases like CO2, forming reversible carbamate or bicarbonate compounds, enabling selective gas capture through chemical bonds. Physical scrubbing, in contrast, depends on the solubility and partial pressure differences of gases in solvents, capturing gases via physical dissolution without chemical reaction. Understanding these chemical mechanisms is crucial for optimizing your gas separation process, as amine scrubbing offers high selectivity and efficiency for acidic gases, while physical scrubbing suits applications with high-pressure and high-gas-concentration streams.
Efficiency in CO₂ Removal
Amine scrubbing offers high efficiency in CO2 removal, achieving capture rates up to 90-99% due to its chemical absorption process that selectively reacts with CO2 molecules. Physical scrubbing relies on dissolving CO2 in a solvent based on pressure and temperature differences, generally providing lower removal efficiency, especially at low CO2 concentrations. Your choice between these methods should consider the specific CO2 concentration and operational conditions to optimize capture performance.
Operating Conditions and Requirements
Amine scrubbing operates efficiently at lower temperatures around 40-60degC and requires steam for solvent regeneration, making it energy-intensive but highly selective for acid gas removal. Physical scrubbing functions best under high pressure and low temperature conditions, typically below 50degC, relying on solvent solubility rather than chemical reactions for gas capture, thus requiring less energy for regeneration. The choice between these methods depends on gas composition, pressure, and energy availability, with amine scrubbing favored for low-pressure, high CO2 concentrations and physical scrubbing preferred in high-pressure environments.
Capital and Operational Costs
Amine scrubbing involves higher capital costs due to complex equipment and corrosion-resistant materials, while physical scrubbing typically requires less expensive infrastructure. Operational costs for amine scrubbing include significant energy consumption for solvent regeneration and frequent chemical replacement, whereas physical scrubbing has lower energy demands but may require costly solvent recovery and maintenance. Your choice between these technologies should consider the trade-off between upfront investment and ongoing operational expenses based on the scale and composition of the gas stream.
Environmental Impact and By-products
Amine scrubbing produces hazardous by-products such as amine degradation compounds and nitrosamines, contributing to soil and water contamination risks. Physical scrubbing systems generate fewer toxic by-products, relying primarily on reversible physical absorption, which minimizes chemical waste and reduces environmental hazards. Both methods require appropriate waste management strategies, but amine scrubbing's chemical nature leads to higher potential environmental impact due to toxic discharge and solvent degradation.
Application Suitability and Industry Usage
Amine scrubbing is highly effective for removing acidic gases like CO2 and H2S from natural gas and refinery gas streams, making it suitable for chemical processing and petroleum industries. Physical scrubbing excels in high-pressure gas streams with low acid gas concentrations, commonly used in syngas cleanup and petrochemical operations. Your choice between these methods depends on gas composition, operating pressure, and industry-specific requirements.
Future Trends and Innovations in Gas Scrubbing
Future trends in gas scrubbing emphasize advanced amine formulations that reduce energy consumption and enhance CO2 capture efficiency, while innovations in physical scrubbing prioritize solvent regeneration and capacity improvements for high-pressure gas streams. Your industrial applications benefit from hybrid systems combining amine and physical scrubbing to optimize environmental performance and operational costs. Emerging technologies also explore membrane integration and advanced process controls to further refine gas treatment processes.
Amine scrubbing vs physical scrubbing Infographic
 libmatt.com
libmatt.com