Chemisorption involves the formation of strong chemical bonds between the adsorbate and surface, resulting in higher specificity and typically irreversible attachment. Your understanding of these distinctions can help optimize processes in catalysis, sensors, and surface coating applications.
Table of Comparison
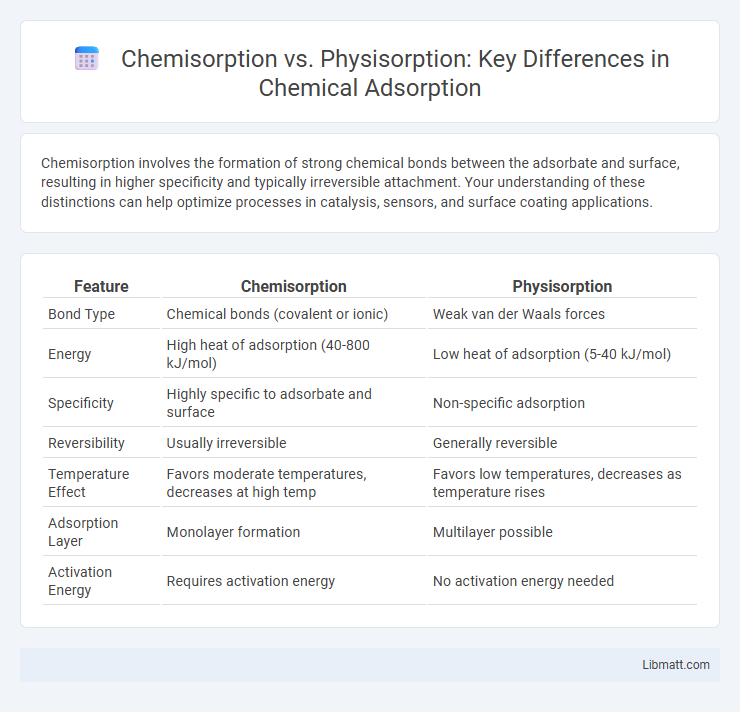
| Feature | Chemisorption | Physisorption |
|---|---|---|
| Bond Type | Chemical bonds (covalent or ionic) | Weak van der Waals forces |
| Energy | High heat of adsorption (40-800 kJ/mol) | Low heat of adsorption (5-40 kJ/mol) |
| Specificity | Highly specific to adsorbate and surface | Non-specific adsorption |
| Reversibility | Usually irreversible | Generally reversible |
| Temperature Effect | Favors moderate temperatures, decreases at high temp | Favors low temperatures, decreases as temperature rises |
| Adsorption Layer | Monolayer formation | Multilayer possible |
| Activation Energy | Requires activation energy | No activation energy needed |
Introduction to Adsorption Phenomena
Adsorption phenomena involve the accumulation of molecules on a surface, where chemisorption forms strong chemical bonds with the substrate, resulting in higher activation energy and specificity. Physisorption relies on weaker van der Waals forces, allowing reversible attachment with lower heat of adsorption and faster equilibrium. Understanding these differences helps you optimize surface interactions for applications in catalysis, sensors, and material science.
Defining Chemisorption
Chemisorption involves the formation of strong chemical bonds between adsorbate molecules and the surface atoms of an adsorbent, resulting in a monolayer adsorption. It typically requires higher activation energy and occurs at elevated temperatures, distinguishing it from physisorption, which relies on weaker van der Waals forces. The specificity and irreversibility of chemisorption make it critical in catalysis and surface reactions.
Understanding Physisorption
Physisorption involves the adsorption of molecules onto a surface through weak van der Waals forces, resulting in reversible and typically low-energy interactions. This process occurs at lower temperatures and is characterized by multilayer adsorption without significant alteration of the adsorbate's chemical structure. Understanding physisorption is essential for optimizing applications like gas storage, catalysis, and surface coating where adsorbate mobility and desorption behavior are critical.
Mechanisms of Chemisorption
Chemisorption involves the formation of strong chemical bonds between adsorbate molecules and the surface atoms of the adsorbent, typically through covalent or ionic interactions. This process is highly specific, often irreversible, and requires an activation energy that leads to the creation of a new electronic configuration at the interface. Your understanding of these mechanisms can enhance the design of catalysts and sensors by optimizing surface interactions at the molecular level.
Mechanisms of Physisorption
Physisorption occurs through weak van der Waals forces between the adsorbate and the surface, resulting in multilayer adsorption without significant electron exchange. This process is typically characterized by low enthalpy changes, reversible binding, and rapid adsorption-desorption kinetics. Surface temperature and pressure strongly influence the extent of physisorption, with increased adsorption at lower temperatures and higher pressures.
Key Differences Between Chemisorption and Physisorption
Chemisorption involves the formation of strong chemical bonds between the adsorbate and the surface, resulting in higher adsorption energy and specificity, whereas physisorption relies on weaker van der Waals forces with lower energy and is generally reversible. Chemisorption typically occurs at higher temperatures and is characterized by monolayer coverage, while physisorption can happen at lower temperatures with multilayer adsorption possible. Understanding these key differences enhances your ability to select the appropriate adsorption process for applications like catalysis or gas storage.
Factors Influencing Chemisorption and Physisorption
Surface temperature and pressure significantly impact chemisorption by altering the activation energy required for bond formation between adsorbate and substrate. In contrast, physisorption is primarily influenced by van der Waals forces and is enhanced at lower temperatures and higher pressures to maximize weak adsorbate-substrate interactions. Your choice of material and environmental conditions will determine which adsorption process dominates based on these factors.
Applications of Chemisorption
Chemisorption plays a crucial role in industrial catalysis, where its strong chemical bonding facilitates efficient reaction mechanisms on catalyst surfaces, enhancing processes like hydrogenation and oxidation. Its application extends to sensor technology, where specific chemical interactions improve sensitivity and selectivity for detecting gases or biomolecules. Your ability to harness chemisorption can significantly optimize performance in fuel cells, heterogeneous catalysis, and surface functionalization for advanced materials.
Applications of Physisorption
Physisorption is widely used in applications such as gas storage, catalyst support, and separation processes due to its reversible nature and weak van der Waals forces. Activated carbon, zeolites, and metal-organic frameworks utilize physisorption for efficient adsorption of gases and vapors in industrial and environmental settings. Your understanding of physisorption helps optimize material selection for filtration, sensor design, and cryogenic gas recovery.
Conclusion: Choosing Between Chemisorption and Physisorption
Choosing between chemisorption and physisorption depends on the desired bond strength, temperature stability, and reversibility of the adsorption process. Chemisorption involves strong chemical bonds with higher activation energy and is preferred for catalytic reactions requiring specificity and permanence. Physisorption, characterized by weak van der Waals forces and low activation energy, suits applications needing reversible adsorption and operation at lower temperatures.
Chemisorption vs physisorption Infographic
 libmatt.com
libmatt.com