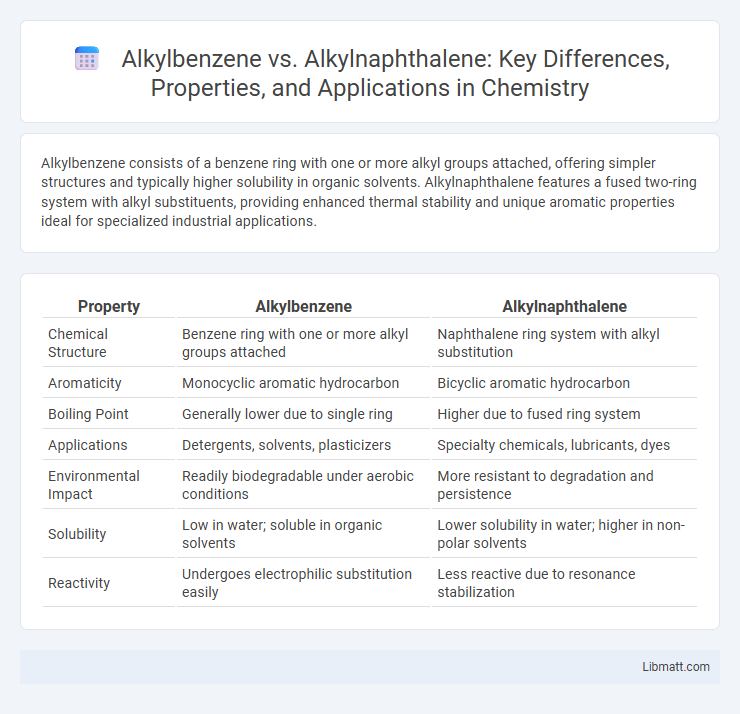
Alkylbenzene consists of a benzene ring with one or more alkyl groups attached, offering simpler structures and typically higher solubility in organic solvents. Alkylnaphthalene features a fused two-ring system with alkyl substituents, providing enhanced thermal stability and unique aromatic properties ideal for specialized industrial applications.
Table of Comparison
| Property | Alkylbenzene | Alkylnaphthalene |
|---|---|---|
| Chemical Structure | Benzene ring with one or more alkyl groups attached | Naphthalene ring system with alkyl substitution |
| Aromaticity | Monocyclic aromatic hydrocarbon | Bicyclic aromatic hydrocarbon |
| Boiling Point | Generally lower due to single ring | Higher due to fused ring system |
| Applications | Detergents, solvents, plasticizers | Specialty chemicals, lubricants, dyes |
| Environmental Impact | Readily biodegradable under aerobic conditions | More resistant to degradation and persistence |
| Solubility | Low in water; soluble in organic solvents | Lower solubility in water; higher in non-polar solvents |
| Reactivity | Undergoes electrophilic substitution easily | Less reactive due to resonance stabilization |
Introduction to Alkylbenzene and Alkylnaphthalene
Alkylbenzene and alkylnaphthalene are aromatic hydrocarbons widely used as intermediates in the chemical industry, particularly in detergents and lubricants. Alkylbenzene features a benzene ring with one or more alkyl groups attached, offering good solubility and biodegradability, while alkylnaphthalene consists of a naphthalene ring system substituted with alkyl chains, providing higher thermal stability and viscosity. Understanding the differences in molecular structure and properties helps optimize your choice for specific industrial applications.
Chemical Structure Comparison
Alkylbenzenes consist of a benzene ring attached to one or more alkyl groups, featuring a single aromatic ring with sp2 hybridized carbon atoms. Alkylnaphthalenes contain two fused benzene rings in a naphthalene structure with alkyl substitutions, resulting in a more complex polycyclic aromatic hydrocarbon with extended conjugation. Your choice between these compounds depends on the desired chemical properties influenced by ring fusion and alkyl chain placement.
Physical Properties Overview
Alkylbenzenes typically exhibit lower melting points and densities compared to alkylnaphthalenes, due to their simpler monocyclic aromatic structure. Alkylnaphthalenes have higher molecular weights and greater thermal stability, resulting in increased viscosity and boiling points. Your choice between these compounds depends on the desired physical properties for applications like lubricants or surfactants.
Synthesis Methods
Alkylbenzene synthesis primarily involves Friedel-Crafts alkylation, where benzene reacts with alkyl halides or alkenes using Lewis acid catalysts like AlCl3, enabling selective monoalkylation. Conversely, alkylnaphthalene synthesis typically employs similar Friedel-Crafts approaches but requires controlled conditions to favor substitution on the naphthalene ring, often using milder Lewis acids to prevent polyalkylation and preserve aromaticity. Advanced methods for both compounds include catalytic alkylation with zeolites or solid acid catalysts, enhancing selectivity and environmental sustainability.
Industrial Applications
Alkylbenzene is widely used in the production of linear alkylbenzene sulfonates (LAS), which serve as key surfactants in detergents and cleaning products due to their excellent biodegradability and detergency. Alkylnaphthalene, on the other hand, finds significant application in the manufacture of high-performance lubricants and specialty chemicals, offering superior thermal stability and antioxidation properties. Understanding the differences between these compounds can optimize Your choice of raw materials for industrial formulations requiring either effective surfactants or advanced lubricant additives.
Environmental Impact Assessment
Alkylbenzene and alkylnaphthalene exhibit distinct environmental impacts assessed through biodegradability and toxicity studies; alkylbenzene tends to have higher biodegradability rates, leading to lower persistence in aquatic ecosystems compared to alkylnaphthalene, which poses greater risks due to its polycyclic aromatic structure and resistance to microbial degradation. Your choice in industrial or laboratory applications should consider alkylnaphthalene's potential for bioaccumulation and long-term environmental persistence, which can result in prolonged ecological harm. Regulatory guidelines often prioritize alkylbenzene's comparatively lower environmental footprint, influencing decisions in environmental risk management and pollutant control strategies.
Performance in Lubricant Formulations
Alkylbenzene exhibits superior antioxidation properties and thermal stability, enhancing lubricant performance under high-temperature conditions. Alkylnaphthalene offers excellent solvency and detergency, improving additive compatibility and cleanliness in engine oils. Both contribute to enhanced viscosity index and oxidation resistance, but alkylbenzene typically provides better overall oxidative stability in lubricant formulations.
Cost and Availability
Alkylbenzenes generally offer lower cost and wider availability due to their simpler structure and extensive production in the petrochemical industry. Alkylnaphthalenes, with more complex two-ring aromatic systems, tend to be more expensive and less readily available, often utilized in specialized applications. Your choice depends on budget constraints and the specific performance requirements of your application.
Safety and Handling Considerations
Alkylbenzenes generally have lower toxicity and are easier to handle compared to alkylnaphthalenes, which pose higher health risks due to their potential carcinogenic properties. Proper personal protective equipment (PPE) and adequate ventilation are essential when working with these compounds to minimize exposure and inhalation hazards. Understanding the specific material safety data sheets (MSDS) for alkylbenzene and alkylnaphthalene ensures your safety by guiding appropriate storage and spill response procedures.
Future Trends in Aromatic Hydrocarbons
Alkylbenzene and alkylnaphthalene are critical aromatic hydrocarbons in advanced lubrication and surfactant formulations, with alkylbenzene favored for its biodegradability and alkylnaphthalene prized for thermal stability and higher molecular weight. Future trends emphasize enhancing bio-based production methods using sustainable feedstocks and catalytic processes to minimize environmental impact. Innovations in molecular engineering aim to optimize performance characteristics such as viscosity index and oxidation resistance, driving demand for tailored aromatic hydrocarbons in automotive and industrial applications.
Alkylbenzene vs alkylnaphthalene Infographic
 libmatt.com
libmatt.com