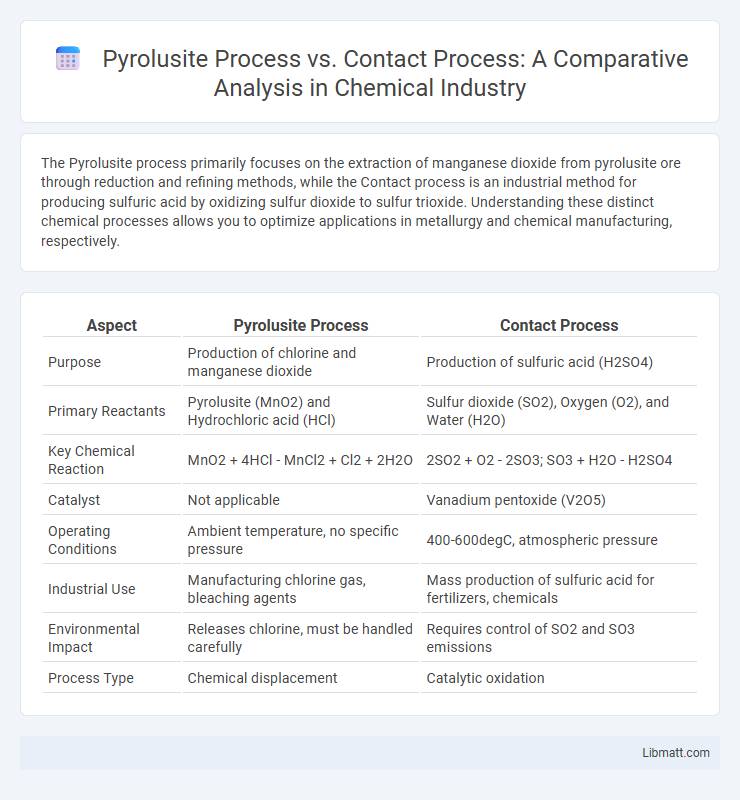
The Pyrolusite process primarily focuses on the extraction of manganese dioxide from pyrolusite ore through reduction and refining methods, while the Contact process is an industrial method for producing sulfuric acid by oxidizing sulfur dioxide to sulfur trioxide. Understanding these distinct chemical processes allows you to optimize applications in metallurgy and chemical manufacturing, respectively.
Table of Comparison
| Aspect | Pyrolusite Process | Contact Process |
|---|---|---|
| Purpose | Production of chlorine and manganese dioxide | Production of sulfuric acid (H2SO4) |
| Primary Reactants | Pyrolusite (MnO2) and Hydrochloric acid (HCl) | Sulfur dioxide (SO2), Oxygen (O2), and Water (H2O) |
| Key Chemical Reaction | MnO2 + 4HCl - MnCl2 + Cl2 + 2H2O | 2SO2 + O2 - 2SO3; SO3 + H2O - H2SO4 |
| Catalyst | Not applicable | Vanadium pentoxide (V2O5) |
| Operating Conditions | Ambient temperature, no specific pressure | 400-600degC, atmospheric pressure |
| Industrial Use | Manufacturing chlorine gas, bleaching agents | Mass production of sulfuric acid for fertilizers, chemicals |
| Environmental Impact | Releases chlorine, must be handled carefully | Requires control of SO2 and SO3 emissions |
| Process Type | Chemical displacement | Catalytic oxidation |
Introduction to Pyrolusite Process and Contact Process
The Pyrolusite process is primarily utilized for manufacturing high-purity manganese dioxide used in dry cell batteries, relying on the natural mineral pyrolusite as its chief raw material. The Contact process, on the other hand, is an industrial method for producing sulfuric acid by oxidizing sulfur dioxide to sulfur trioxide using a vanadium pentoxide catalyst at high temperatures. Both processes are essential in chemical manufacturing, with the Pyrolusite process centered on manganese compound extraction and the Contact process focusing on large-scale acid production.
Historical Background of Both Processes
The Pyrolusite process, dating back to the 19th century, was one of the earliest industrial methods for producing chlorine by reacting hydrochloric acid with pyrolusite (MnO2), a manganese dioxide ore. The Contact process emerged later in the early 20th century as a more efficient and commercially viable method for producing sulfuric acid, involving the catalytic oxidation of sulfur dioxide using vanadium pentoxide catalyst under controlled temperature conditions. Both processes reflect the advancement in chemical engineering, with the Pyrolusite process representing an initial step towards industrial chlorine production and the Contact process marking significant enhancement in sulfuric acid synthesis.
Chemical Reactions Involved
The pyrolusite process involves the oxidation of manganese dioxide (MnO2) as a key reactant to produce chlorine gas (Cl2) through the reaction MnO2 + 4HCl - MnCl2 + 2H2O + Cl2. The contact process primarily relies on the catalytic oxidation of sulfur dioxide (SO2) to sulfur trioxide (SO3) using vanadium pentoxide (V2O5) as a catalyst, following the reaction 2SO2 + O2 - 2SO3. These chemical reactions underpin the efficient production of chlorine from pyrolusite and sulfuric acid from sulfur dioxide in the contact process.
Raw Materials and Their Availability
Pyrolusite process primarily uses manganese dioxide, a naturally abundant ore commonly found in large deposits worldwide, making it widely accessible for industrial applications. Contact process relies on sulfur dioxide derived from burning sulfur or roasting sulfide ores, with raw materials like elemental sulfur or pyrite being regionally available but sometimes limited by mining constraints. The availability of pyrolusite ensures a relatively stable supply chain for manganese-based products, whereas the contact process depends on sulfur sources that can fluctuate based on geological and economic factors.
Process Flow and Operational Steps
The pyrolusite process involves the electrolytic reduction of manganese dioxide (pyrolusite) to produce high-purity manganese metal, passing through ore preparation, electrolysis in an electrolytic cell, and purification stages. The contact process converts sulfur dioxide (SO2) to sulfur trioxide (SO3) using a vanadium pentoxide catalyst in a multi-stage reactor system, including gas preparation, catalytic conversion, and absorption. Your choice between these processes depends on the desired chemical output and specific industrial requirements for process flow and operational control.
Efficiency and Yield Comparison
The pyrolusite process demonstrates higher efficiency for manganese dioxide extraction, commonly achieving yield rates exceeding 90%, which is essential for battery and steel production industries. In contrast, the contact process, primarily used for sulfuric acid synthesis, achieves optimal conversion efficiencies around 98-99% due to advanced catalyst technology and precise temperature controls. While the contact process offers superior yield in producing sulfur trioxide, the pyrolusite process excels in raw material conversion efficiency for manganese-related applications.
Environmental Impact and Sustainability
The pyrolusite process relies on natural manganese dioxide, which is abundant and enables a relatively low-energy production of chlorine and sodium hydroxide but may lead to manganese residue disposal challenges affecting soil and water quality. The contact process for sulfuric acid production typically emits sulfur dioxide and requires strict emission controls to prevent acid rain, making it critical to implement scrubbers and gas cleaning systems to minimize environmental harm. Both processes demand careful resource management and pollution mitigation to enhance sustainability, but the contact process involves more complex environmental compliance due to sulfur oxide emissions.
Industrial Applications and End Products
The pyrolusite process primarily produces manganese dioxide, widely used in dry cell batteries and as a depolarizer in steel production. The contact process is essential for manufacturing sulfuric acid, a critical chemical in fertilizer production, petroleum refining, and chemical synthesis industries. While pyrolusite targets manganese-based products, the contact process focuses on sulfuric acid, underscoring their distinct industrial applications and end product outputs.
Cost Analysis and Economic Considerations
The pyrolusite process generally incurs lower raw material costs due to abundant availability of pyrolusite ore, making it economically favorable for large-scale manganese dioxide production. In contrast, the contact process demands higher capital investment and operational expenses because of stringent temperature control and catalyst regeneration requirements. Your choice depends on balancing initial outlays against continuous operational costs and production scale efficiencies.
Future Prospects and Technological Advancements
The pyrolusite process, central to manganese dioxide production, faces future advancements through enhanced catalytic efficiency and eco-friendly extraction techniques, supporting battery and steel industries amid growing demand. In contrast, the contact process for sulfuric acid synthesis is evolving with innovations in catalyst materials and emission reduction technologies to meet stricter environmental regulations and industrial scalability. Both processes demonstrate significant potential for integration with digital monitoring systems and energy-efficient operations, driving sustainability and cost-effectiveness in chemical manufacturing.
pyrolusite process vs contact process Infographic
 libmatt.com
libmatt.com