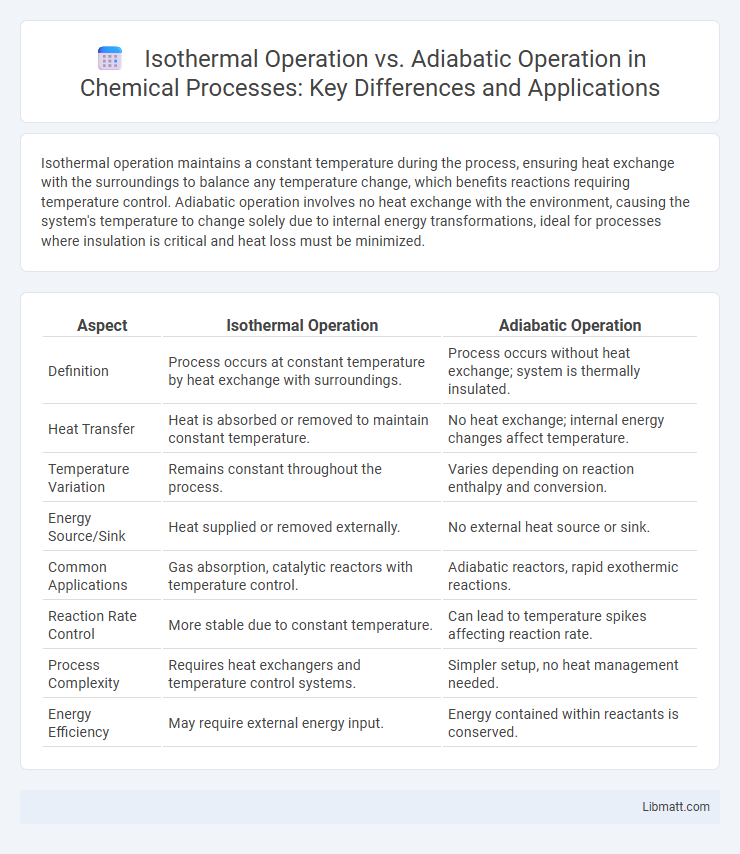
Isothermal operation maintains a constant temperature during the process, ensuring heat exchange with the surroundings to balance any temperature change, which benefits reactions requiring temperature control. Adiabatic operation involves no heat exchange with the environment, causing the system's temperature to change solely due to internal energy transformations, ideal for processes where insulation is critical and heat loss must be minimized.
Table of Comparison
| Aspect | Isothermal Operation | Adiabatic Operation |
|---|---|---|
| Definition | Process occurs at constant temperature by heat exchange with surroundings. | Process occurs without heat exchange; system is thermally insulated. |
| Heat Transfer | Heat is absorbed or removed to maintain constant temperature. | No heat exchange; internal energy changes affect temperature. |
| Temperature Variation | Remains constant throughout the process. | Varies depending on reaction enthalpy and conversion. |
| Energy Source/Sink | Heat supplied or removed externally. | No external heat source or sink. |
| Common Applications | Gas absorption, catalytic reactors with temperature control. | Adiabatic reactors, rapid exothermic reactions. |
| Reaction Rate Control | More stable due to constant temperature. | Can lead to temperature spikes affecting reaction rate. |
| Process Complexity | Requires heat exchangers and temperature control systems. | Simpler setup, no heat management needed. |
| Energy Efficiency | May require external energy input. | Energy contained within reactants is conserved. |
Introduction to Isothermal and Adiabatic Operations
Isothermal operation maintains a constant temperature throughout the process by allowing heat exchange with the surroundings, enhancing energy efficiency in chemical reactions or phase changes. Adiabatic operation, by contrast, involves no heat transfer, causing temperature changes within the system that influence reaction rates and thermodynamic properties. Your choice between isothermal and adiabatic conditions determines process control, energy requirements, and overall system performance in industrial applications.
Fundamental Thermodynamic Principles
Isothermal operation maintains a constant temperature by allowing heat exchange with the surroundings, ensuring the system's internal energy remains stable while work is performed. Adiabatic operation involves no heat transfer, causing all work input or output to directly affect the system's internal energy, resulting in temperature changes. The fundamental thermodynamic distinction lies in the heat transfer process: isothermal processes require reversible heat exchange to maintain equilibrium, whereas adiabatic processes are insulated, leading to internal energy changes governed by the first law of thermodynamics.
Key Differences Between Isothermal and Adiabatic Processes
Isothermal operation maintains a constant temperature throughout the process by allowing heat exchange with the surroundings, whereas adiabatic operation occurs without any heat transfer, causing temperature changes due to work done on or by the system. Isothermal processes generally involve slower rates to ensure thermal equilibrium, while adiabatic processes are rapid and insulated to prevent heat loss or gain. The key distinction lies in heat transfer: isothermal processes require heat flow to maintain temperature, whereas adiabatic processes occur in thermally isolated conditions with internal energy variations manifesting as temperature changes.
Temperature and Pressure Behavior in Each Operation
Isothermal operation maintains a constant temperature by allowing heat exchange with the surroundings, resulting in a gradual pressure change as the system adapts to volume or phase changes. In contrast, adiabatic operation involves no heat transfer, causing the temperature to fluctuate significantly along with pressure due to internal energy changes during compression or expansion. Your system's choice between these operations affects temperature stability and pressure response critical for process control and efficiency.
Heat Transfer Characteristics: Isothermal vs Adiabatic
Isothermal operation maintains a constant temperature by allowing continuous heat transfer with the surroundings, ensuring thermal equilibrium throughout the process. Adiabatic operation, in contrast, involves no heat exchange, causing temperature fluctuations due to internal energy changes and work done on or by the system. Your system's choice between isothermal and adiabatic conditions significantly impacts heat transfer management, thermal efficiency, and process stability.
Energy Efficiency and Work Output Comparisons
Isothermal operation maximizes energy efficiency by maintaining constant temperature, allowing continuous heat exchange that minimizes energy loss, resulting in higher work output per cycle. Adiabatic operation, characterized by no heat transfer, often generates higher peak work output but with lower overall energy efficiency due to temperature fluctuations and increased entropy. Your choice between these operational modes depends on prioritizing sustained efficiency or maximum instantaneous power.
Common Industrial Applications
Isothermal operation is prevalent in chemical reactors and gas separation processes where temperature control is critical to maintain reaction rates and product quality. Adiabatic operation is commonly used in combustion engines and certain compressors where no heat exchange with the environment allows for efficient energy conversion and rapid temperature changes. Industries such as petrochemical refining and air compression frequently utilize both methods based on process requirements and thermal management strategies.
Equipment Design Considerations
Isothermal operation requires equipment designed for efficient heat exchange to maintain constant temperature, often involving heat exchangers or jackets to remove or add heat continuously. Adiabatic operation demands insulation and robust materials to handle temperature changes without heat loss, focusing on minimizing thermal gradients within the system. Your choice affects equipment size, material selection, and safety measures to ensure optimal performance under specific thermal conditions.
Advantages and Disadvantages of Each Approach
Isothermal operation maintains constant temperature by allowing heat exchange with the surroundings, resulting in higher energy efficiency and reduced thermal stress on equipment but requires complex heat management systems. Adiabatic operation involves no heat transfer, simplifying system design and enabling rapid process cycles, yet it often leads to temperature fluctuations that can cause thermal degradation or lower product quality. Choosing between these approaches depends on balancing energy efficiency, equipment durability, process speed, and quality requirements.
Choosing the Right Operation for Your Process
Isothermal operation maintains a constant temperature by exchanging heat with the surroundings, ideal for processes requiring precise temperature control and minimal thermal stress. Adiabatic operation occurs without heat exchange, suitable for reactions where heat retention increases efficiency or drives the process forward. Understanding your process's thermal sensitivity and energy requirements helps determine whether isothermal or adiabatic conditions best optimize performance and product quality.
isothermal operation vs adiabatic operation Infographic
 libmatt.com
libmatt.com