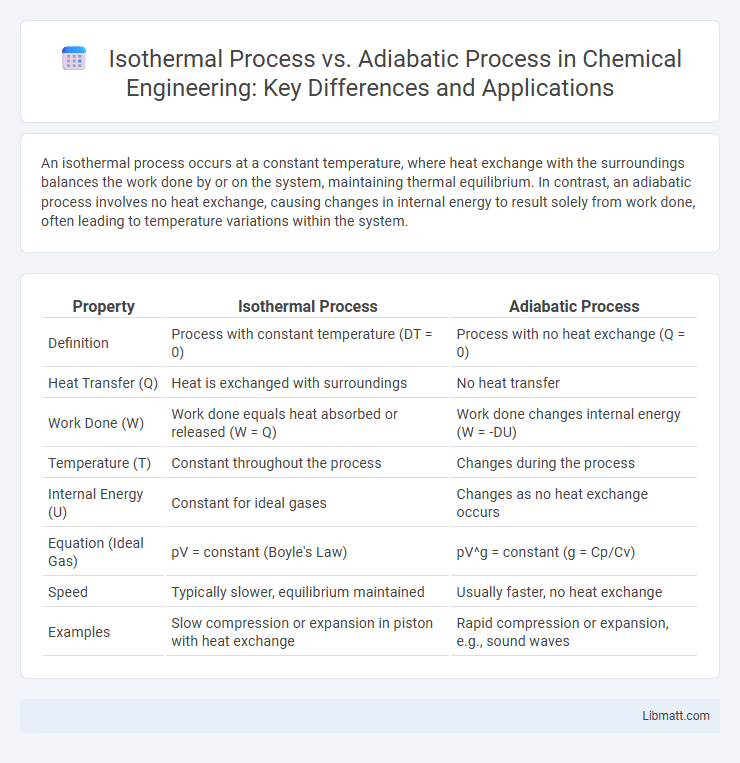
An isothermal process occurs at a constant temperature, where heat exchange with the surroundings balances the work done by or on the system, maintaining thermal equilibrium. In contrast, an adiabatic process involves no heat exchange, causing changes in internal energy to result solely from work done, often leading to temperature variations within the system.
Table of Comparison
| Property | Isothermal Process | Adiabatic Process |
|---|---|---|
| Definition | Process with constant temperature (DT = 0) | Process with no heat exchange (Q = 0) |
| Heat Transfer (Q) | Heat is exchanged with surroundings | No heat transfer |
| Work Done (W) | Work done equals heat absorbed or released (W = Q) | Work done changes internal energy (W = -DU) |
| Temperature (T) | Constant throughout the process | Changes during the process |
| Internal Energy (U) | Constant for ideal gases | Changes as no heat exchange occurs |
| Equation (Ideal Gas) | pV = constant (Boyle's Law) | pV^g = constant (g = Cp/Cv) |
| Speed | Typically slower, equilibrium maintained | Usually faster, no heat exchange |
| Examples | Slow compression or expansion in piston with heat exchange | Rapid compression or expansion, e.g., sound waves |
Introduction to Thermodynamic Processes
Isothermal processes occur at a constant temperature, allowing heat exchange with the surroundings while maintaining thermal equilibrium. Adiabatic processes involve no heat transfer, resulting in temperature changes due to work done on or by the system. Understanding these thermodynamic processes is crucial for analyzing energy transformations in engines, refrigerators, and various industrial applications.
Defining Isothermal and Adiabatic Processes
Isothermal processes occur when a system's temperature remains constant while heat exchange with the surroundings takes place, causing changes in pressure and volume. Adiabatic processes involve no heat transfer, resulting in temperature changes due solely to work done on or by the system. These fundamental distinctions are crucial for analyzing thermodynamic cycles and energy transformations in gases.
Key Differences Between Isothermal and Adiabatic Processes
Isothermal processes maintain a constant temperature throughout the transformation, allowing heat exchange with the surroundings, whereas adiabatic processes occur without any heat transfer, resulting in temperature changes within the system. In isothermal processes, the internal energy remains constant, while in adiabatic processes, changes in internal energy correspond directly to work done on or by the system. The key difference lies in heat exchange: isothermal processes involve thermal interaction, whereas adiabatic processes are thermally insulated.
The Science Behind Isothermal Processes
Isothermal processes maintain a constant temperature by allowing heat exchange with the surroundings, enabling the system's internal energy to remain unchanged as work is done. This process is governed by the ideal gas law, where pressure inversely varies with volume while temperature stays steady. The science behind isothermal processes highlights the equilibrium between heat absorbed and work performed, distinguishing it from adiabatic processes where no heat exchange occurs.
The Science Behind Adiabatic Processes
Adiabatic processes involve the transfer of energy solely through work without any heat exchange with the surroundings, governed by the first law of thermodynamics. During an adiabatic process, the temperature of the system changes due to pressure and volume variations, as energy remains conserved within the system. Understanding the science behind adiabatic processes is crucial for applications in atmospheric science, thermodynamics, and engineering systems where heat insulation is significant.
Real-World Examples of Isothermal Processes
Isothermal processes occur in systems like phase changes in water at boiling or freezing points, where temperature remains constant despite heat exchange. Industrial applications include the operation of heat exchangers and refrigerators, where gases expand or compress slowly to maintain thermal equilibrium. In contrast, adiabatic processes happen rapidly without heat transfer, exemplified by the compression of air in diesel engines or the expansion of gas in a piston during power strokes.
Real-World Examples of Adiabatic Processes
Adiabatic processes occur in real-world scenarios such as the rapid compression or expansion of gases in internal combustion engines and the rising air parcels that cool and condense to form clouds in atmospheric science. Unlike isothermal processes, which maintain constant temperature through heat exchange with the environment, adiabatic processes involve no heat transfer, resulting in temperature changes solely due to pressure variations. Industrial applications also include gas turbines and refrigeration cycles, where adiabatic expansion and compression are fundamental for efficient energy transfer.
Applications in Engineering and Industry
Isothermal processes are essential in industries requiring temperature control during gas expansion or compression, such as refrigeration cycles and chemical reactors, where heat exchange maintains constant temperature. Adiabatic processes are crucial in applications like gas turbines and compressors, where rapid compression or expansion occurs without heat transfer, maximizing efficiency and mechanical work output. Your engineering designs can benefit from selecting the appropriate process to optimize thermal management and energy efficiency in industrial systems.
Isothermal vs Adiabatic: Efficiency and Limitations
Isothermal processes maintain constant temperature by allowing heat exchange with the surroundings, resulting in higher theoretical efficiency in ideal gas cycles compared to adiabatic processes, which occur without heat transfer and rely solely on internal energy changes. Adiabatic processes are limited by rapid execution requirements to prevent heat exchange, often leading to less practical efficiency due to real-world friction and non-ideal behavior. Efficiency in thermodynamic cycles such as the Carnot cycle is maximized by isothermal expansion and compression steps, while adiabatic steps are crucial for work output but have efficiency constraints due to entropy generation in real systems.
Summary and Key Takeaways
Isothermal processes maintain a constant temperature, allowing heat exchange with the surroundings, while adiabatic processes involve no heat transfer, causing temperature changes within the system. Understanding these distinctions helps optimize thermal systems like engines and refrigerators by controlling energy flow and efficiency. Your choice between isothermal and adiabatic processes depends on whether temperature stability or rapid compression without heat loss is needed.
Isothermal process vs adiabatic process Infographic
 libmatt.com
libmatt.com