Distillation separates components based on differences in boiling points by heating a liquid mixture to collect vaporized fractions, while extraction relies on solubility differences to transfer compounds from one solvent to another. Your choice depends on the nature of the substances involved and the desired purity level.
Table of Comparison
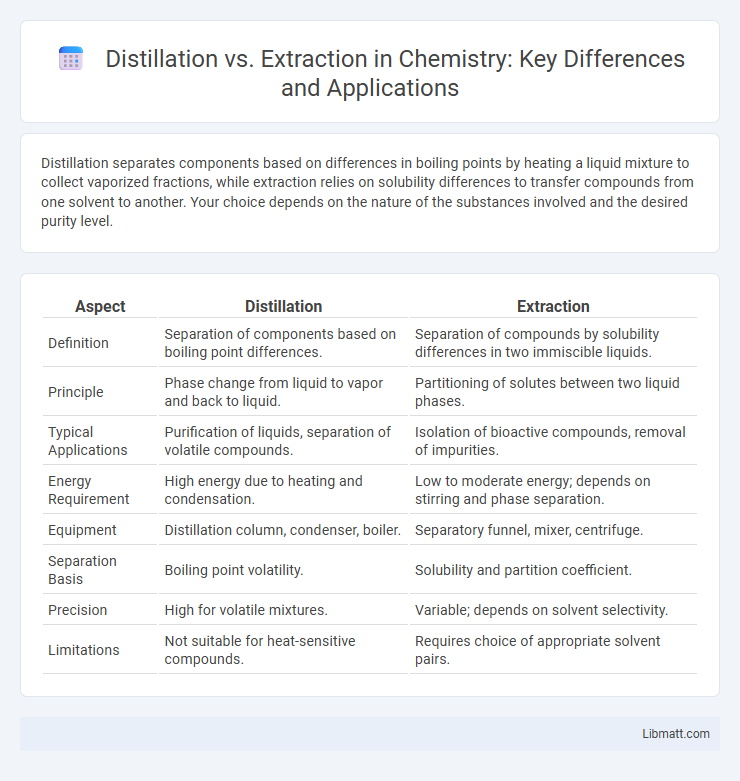
| Aspect | Distillation | Extraction |
|---|---|---|
| Definition | Separation of components based on boiling point differences. | Separation of compounds by solubility differences in two immiscible liquids. |
| Principle | Phase change from liquid to vapor and back to liquid. | Partitioning of solutes between two liquid phases. |
| Typical Applications | Purification of liquids, separation of volatile compounds. | Isolation of bioactive compounds, removal of impurities. |
| Energy Requirement | High energy due to heating and condensation. | Low to moderate energy; depends on stirring and phase separation. |
| Equipment | Distillation column, condenser, boiler. | Separatory funnel, mixer, centrifuge. |
| Separation Basis | Boiling point volatility. | Solubility and partition coefficient. |
| Precision | High for volatile mixtures. | Variable; depends on solvent selectivity. |
| Limitations | Not suitable for heat-sensitive compounds. | Requires choice of appropriate solvent pairs. |
Introduction to Distillation and Extraction
Distillation separates components based on differences in boiling points by heating a liquid mixture to vaporize and then condensing the vapor to obtain purified substances. Extraction uses a solvent to selectively dissolve and separate desired compounds from a mixture, often relying on differences in solubility or polarity. Your choice between distillation and extraction depends on the physical and chemical properties of the components you aim to isolate.
Fundamental Principles of Distillation
Distillation relies on the fundamental principle of separating components based on differences in their boiling points, where a mixture is heated to vaporize the more volatile substances and then condensed to collect the purified component. This phase change exploits volatility disparities, allowing precise separation of liquid mixtures into individual fractions. Understanding these principles enables you to optimize distillation processes for efficient purification in industries such as petrochemicals, pharmaceuticals, and food production.
Fundamental Principles of Extraction
Extraction fundamentally relies on the solubility differences of compounds between two immiscible phases, typically a solid-liquid or liquid-liquid system, to selectively separate desired components. It involves mass transfer driven by concentration gradients, where target substances partition preferentially into a solvent based on polarity, molecular size, and affinity. This technique contrasts distillation, which separates compounds through differences in volatility and boiling points rather than solubility and phase partitioning.
Key Differences Between Distillation and Extraction
Distillation separates components based on differences in boiling points by heating a liquid mixture to vaporize and then condense its components, whereas extraction isolates substances by transferring solutes from one solvent to another based on solubility differences. Distillation is ideal for purifying liquids and separating volatile compounds, while extraction excels in isolating non-volatile compounds and impurities from solid or liquid mixtures. The choice between distillation and extraction depends on factors like the physical state of components, boiling points, and the nature of solvents used.
Applications of Distillation in Industry
Distillation plays a critical role in industries such as petrochemical refining, where it separates crude oil into valuable components like gasoline, diesel, and jet fuel based on boiling points. The pharmaceutical industry relies on distillation to purify solvents and isolate active ingredients, ensuring product quality and safety. Your manufacturing processes benefit from distillation's efficiency in producing high-purity chemicals and essential oils across food, beverage, and cosmetic sectors.
Applications of Extraction in Industry
Extraction in industry is widely applied for separating valuable compounds from complex mixtures, such as obtaining essential oils from plants, recovering metals from ores, and purifying pharmaceuticals. Liquid-liquid extraction is commonly used in chemical manufacturing to isolate specific components based on their solubility differences. Solid-liquid extraction serves crucial roles in food processing, such as extracting caffeine from coffee beans and flavors from natural products.
Advantages and Limitations of Distillation
Distillation offers the advantage of effectively separating mixtures based on differences in boiling points, making it ideal for purifying liquids such as in petroleum refining and alcoholic beverage production. Its limitations include high energy consumption and inefficiency when dealing with compounds that have very close boiling points or are heat-sensitive. You should consider these factors when choosing distillation for your separation needs, as alternative methods like extraction may be more suitable for delicate or closely related substances.
Advantages and Limitations of Extraction
Extraction offers advantages such as selective separation of compounds based on solubility, preserving the integrity of heat-sensitive components that might degrade during distillation. Its limitations include the potential use of large volumes of solvents, which can be costly and environmentally hazardous, as well as slower processing times compared to distillation. Your choice between extraction and distillation should consider factors like compound stability, purity requirements, and operational efficiency.
Choosing Between Distillation and Extraction
Choosing between distillation and extraction depends on the chemical properties and volatility of the components in a mixture. Distillation is optimal for separating liquids with different boiling points through controlled heating and condensation. Extraction is preferred for isolating compounds based on their solubility differences in two immiscible liquids, especially when thermal sensitivity is a concern.
Future Trends in Separation Technologies
Future trends in separation technologies highlight the integration of hybrid distillation-extraction systems to enhance efficiency and selectivity in complex mixtures. Innovations such as membrane-assisted distillation and solvent-resistant membranes are driving breakthroughs in energy reduction and sustainability. Advances in process intensification and automation enable real-time monitoring and adaptive control, optimizing separation performance for industrial applications.
Distillation vs extraction Infographic
 libmatt.com
libmatt.com