Carbon adsorption effectively removes organic compounds, chlorine, and certain chemicals from water by trapping them on activated carbon surfaces, enhancing taste and odor. Ion exchange targets specific ions like calcium, magnesium, and heavy metals by exchanging them with sodium or hydrogen ions, improving water softness and reducing mineral content.
Table of Comparison
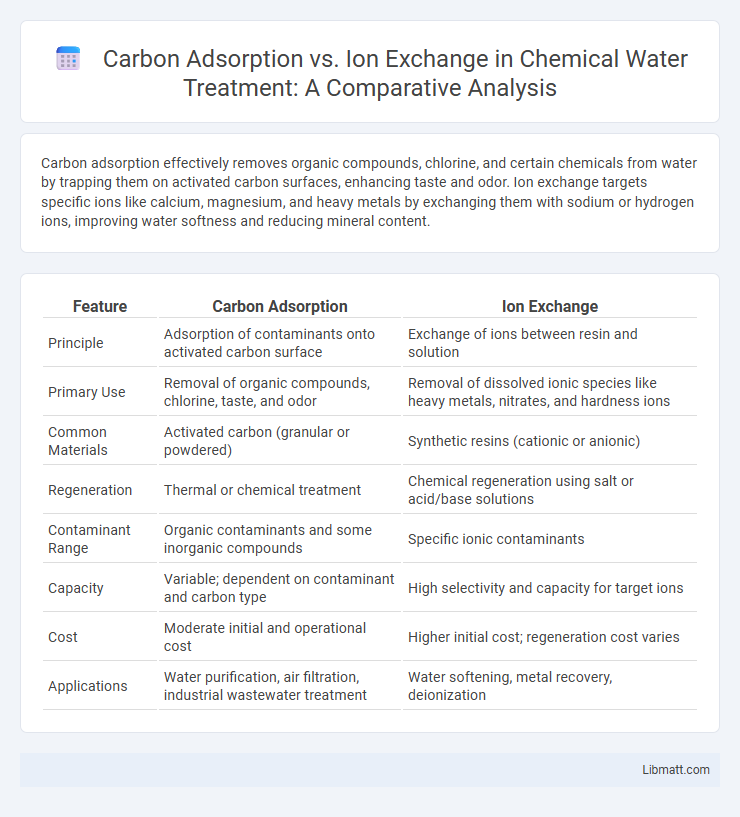
| Feature | Carbon Adsorption | Ion Exchange |
|---|---|---|
| Principle | Adsorption of contaminants onto activated carbon surface | Exchange of ions between resin and solution |
| Primary Use | Removal of organic compounds, chlorine, taste, and odor | Removal of dissolved ionic species like heavy metals, nitrates, and hardness ions |
| Common Materials | Activated carbon (granular or powdered) | Synthetic resins (cationic or anionic) |
| Regeneration | Thermal or chemical treatment | Chemical regeneration using salt or acid/base solutions |
| Contaminant Range | Organic contaminants and some inorganic compounds | Specific ionic contaminants |
| Capacity | Variable; dependent on contaminant and carbon type | High selectivity and capacity for target ions |
| Cost | Moderate initial and operational cost | Higher initial cost; regeneration cost varies |
| Applications | Water purification, air filtration, industrial wastewater treatment | Water softening, metal recovery, deionization |
Introduction to Water Treatment Technologies
Carbon adsorption effectively removes organic contaminants and chlorine from water through porous activated carbon, enhancing taste and odor while reducing harmful compounds. Ion exchange targets dissolved ions such as heavy metals, calcium, and magnesium by exchanging unwanted ions with more benign ones, crucial for softening water and removing hardness. Both technologies play pivotal roles in advanced water treatment systems, addressing different contaminant profiles for improved water quality.
Overview of Carbon Adsorption
Carbon adsorption is a widely used water treatment process that employs activated carbon to remove organic compounds, chlorine, and odors through physical adsorption. The high surface area and porous structure of activated carbon enable it to capture contaminants effectively, improving water taste and safety. Your water purification system can benefit from carbon adsorption by reducing impurities that ion exchange may not target.
Fundamentals of Ion Exchange
Ion exchange operates on the principle of reversible interchange of ions between a solid resin and a liquid, selectively removing unwanted ions from water by replacing them with more desirable ones. This process utilizes ion-exchange resins composed of polymer beads with charged functional groups that attract and hold ions based on charge and size. Your water treatment efficiency depends on factors like resin type, regeneration frequency, and water composition, making ion exchange ideal for applications like water softening and deionization.
Mechanisms of Contaminant Removal
Carbon adsorption removes contaminants through physical adsorption, where pollutants adhere to the porous carbon surface via Van der Waals forces, effectively trapping organic compounds, chlorine, and certain heavy metals. Ion exchange operates by exchanging ions in the contaminant solution with ions attached to a resin, selectively removing dissolved ions such as heavy metals, nitrates, and hardness-causing calcium or magnesium ions. The differing mechanisms make carbon adsorption ideal for organic and chlorine removal, while ion exchange excels in water softening and removal of dissolved inorganic contaminants.
Types of Contaminants Addressed
Carbon adsorption effectively removes organic contaminants, chlorine, volatile organic compounds (VOCs), pesticides, and certain heavy metals by trapping them in the porous carbon material. Ion exchange targets dissolved ions such as hardness minerals (calcium, magnesium), heavy metals (lead, mercury), and nitrates by swapping them with harmless ions like sodium or hydrogen. Your choice depends on whether you need to eliminate organic pollutants or ionic dissolved substances from water.
Efficiency and Performance Comparison
Carbon adsorption effectively removes organic compounds and chlorine from water with high efficiency, particularly in eliminating taste and odor issues. Ion exchange excels in removing dissolved ions such as heavy metals, nitrates, and hardness-causing minerals, providing targeted ion-specific purification. Your choice depends on water quality needs, as carbon adsorption offers broad contaminant removal while ion exchange delivers precise ion reduction.
System Design and Operational Considerations
Carbon adsorption systems involve granular or powdered activated carbon units designed for high surface area contact, requiring periodic regeneration or replacement of carbon media to maintain efficiency. Ion exchange systems incorporate resin beds tailored to target specific ions, necessitating careful monitoring of resin capacity, regeneration cycles, and chemical usage to optimize performance. Both designs demand attention to flow rates, influent water quality, and system maintenance schedules to ensure effective contaminant removal and operational longevity.
Cost Analysis and Economic Factors
Carbon adsorption generally incurs lower initial capital costs compared to ion exchange systems due to simpler design and fewer components. Operating expenses for carbon adsorption include frequent media replacement and energy consumption for regeneration, while ion exchange systems require higher chemical costs and skilled labor for resin regeneration and disposal. Economic factors favor carbon adsorption for treating moderate contamination levels, whereas ion exchange offers cost-effectiveness for high-purity applications despite higher upfront and operational expenses.
Environmental Impact and Sustainability
Carbon adsorption utilizes activated carbon, a porous material often derived from renewable resources, which can be regenerated but eventually requires disposal, posing potential environmental concerns if not managed properly. Ion exchange resins, typically synthetic polymers, can be regenerated and reused multiple times, reducing waste generation, but their production and disposal involve chemicals that may impact ecosystems negatively. Both technologies demand careful lifecycle management to maximize sustainability, with increasing interest in bio-based adsorbents and green resin synthesis to mitigate environmental footprints.
Choosing the Right Technology for Your Needs
Choosing between carbon adsorption and ion exchange depends on the specific contaminants and treatment goals. Carbon adsorption excels at removing organic compounds, chlorine, and taste-odor issues, making it ideal for improving water quality and safety. Ion exchange is more effective for targeting dissolved ions such as hardness minerals, heavy metals, and nitrates, offering precise control over water chemistry for industrial or drinking water applications.
Carbon adsorption vs ion exchange Infographic
 libmatt.com
libmatt.com