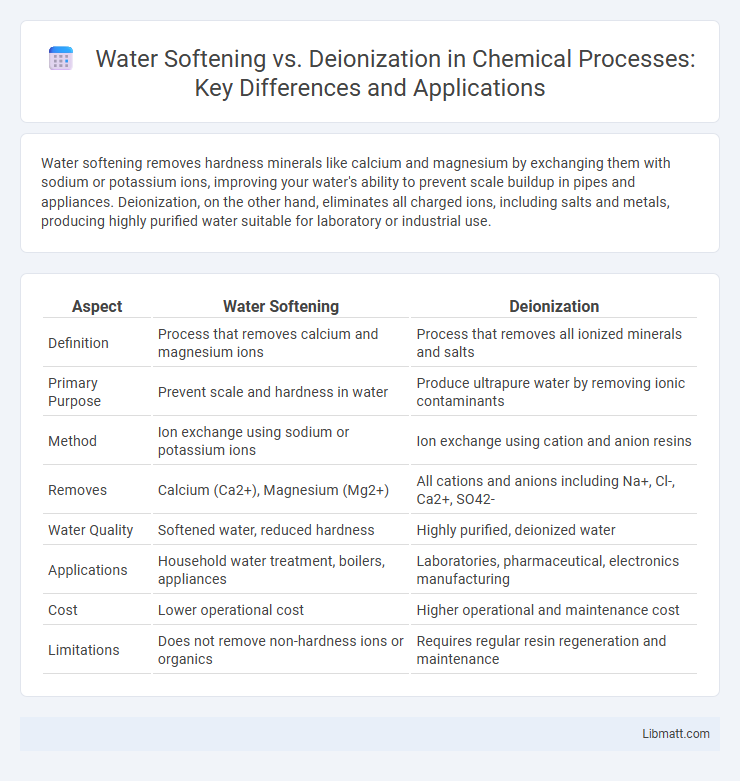
Water softening removes hardness minerals like calcium and magnesium by exchanging them with sodium or potassium ions, improving your water's ability to prevent scale buildup in pipes and appliances. Deionization, on the other hand, eliminates all charged ions, including salts and metals, producing highly purified water suitable for laboratory or industrial use.
Table of Comparison
| Aspect | Water Softening | Deionization |
|---|---|---|
| Definition | Process that removes calcium and magnesium ions | Process that removes all ionized minerals and salts |
| Primary Purpose | Prevent scale and hardness in water | Produce ultrapure water by removing ionic contaminants |
| Method | Ion exchange using sodium or potassium ions | Ion exchange using cation and anion resins |
| Removes | Calcium (Ca2+), Magnesium (Mg2+) | All cations and anions including Na+, Cl-, Ca2+, SO42- |
| Water Quality | Softened water, reduced hardness | Highly purified, deionized water |
| Applications | Household water treatment, boilers, appliances | Laboratories, pharmaceutical, electronics manufacturing |
| Cost | Lower operational cost | Higher operational and maintenance cost |
| Limitations | Does not remove non-hardness ions or organics | Requires regular resin regeneration and maintenance |
Introduction: Understanding Water Softening and Deionization
Water softening primarily removes calcium and magnesium ions to prevent scale buildup, using ion exchange resins with sodium ions. Deionization eliminates nearly all dissolved minerals and ionic impurities by exchanging cations and anions with hydrogen and hydroxide ions, producing highly purified water. Both processes improve water quality but differ significantly in application, cost, and the degree of purification achieved.
What is Water Softening?
Water softening is a process that removes hardness-causing minerals, primarily calcium and magnesium ions, from your water supply to prevent scale buildup in pipes and appliances. This process typically involves ion exchange technology, where hardness ions are replaced with sodium or potassium ions, improving water quality for cleaning and industrial applications. Understanding water softening helps you maintain efficient plumbing systems and extend the lifespan of water-using equipment.
How Does Deionization Work?
Deionization works by removing dissolved ionized minerals and salts from water using ion exchange resins that replace cations and anions with hydrogen and hydroxyl ions, which combine to form pure water. This process effectively eliminates ions such as calcium, magnesium, sodium, and chloride that cause water hardness and conductivity. Your water becomes highly purified, making deionization ideal for applications requiring low-ionic content water, unlike traditional water softening, which only exchanges hardness ions without removing all dissolved solids.
Key Differences Between Water Softening and Deionization
Water softening primarily removes calcium and magnesium ions to prevent scale buildup, while deionization eliminates almost all dissolved mineral ions, including cations and anions, resulting in highly purified water. Softening uses ion-exchange resins that replace hardness ions with sodium or potassium, whereas deionization employs both cation and anion exchange resins for comprehensive ion removal. The main practical difference lies in water softness versus overall purity, with softened water suited for household use and deionized water required for laboratory and industrial applications.
Applications of Water Softening Systems
Water softening systems are primarily used in residential and commercial settings to reduce hardness caused by calcium and magnesium ions, improving appliance efficiency and extending their lifespan. Industries such as hospitality, laundries, and food processing rely on softened water to prevent scale buildup in boilers, cooling towers, and piping systems. These systems enhance water quality for cleaning, bathing, and manufacturing processes by minimizing mineral deposits and improving detergent effectiveness.
Uses of Deionized Water
Deionized water is extensively used in laboratories, pharmaceuticals, and electronics manufacturing due to its high purity and lack of ionic contaminants. It is essential for processes requiring minimal mineral content, such as preparing chemical solutions and rinsing sensitive components. In contrast to softened water, deionized water ensures the elimination of dissolved salts, preventing interference in precise analytical applications.
Water Quality Outcomes: Softened vs Deionized
Water softening primarily removes calcium and magnesium ions, reducing hardness to prevent scale buildup, resulting in improved water quality suitable for household and industrial applications. Deionization eliminates almost all dissolved ions, including cations and anions, producing ultra-pure water ideal for laboratory and pharmaceutical uses. The key difference in water quality outcomes is that softened water retains most minerals except hardness-causing elements, while deionized water achieves near-total ion removal for maximum purity.
Maintenance Requirements and Costs
Water softening systems require regular addition of salt and occasional resin replacement, resulting in moderate maintenance costs typically ranging from $100 to $300 annually. Deionization units demand more frequent resin regeneration or cartridge replacement, leading to higher maintenance expenses often exceeding $500 per year. Your choice depends on balancing lower ongoing costs of softeners with the superior purity but greater upkeep cost of deionization systems.
Selecting the Right Method for Your Needs
Water softening removes hardness-causing minerals like calcium and magnesium to prevent scale buildup, making it ideal for household appliances and plumbing systems. Deionization eliminates all ionized minerals, delivering ultra-pure water suited for laboratory and industrial applications requiring minimal conductivity. Assess your specific water quality requirements and usage to select the right method for your needs, ensuring optimal performance and cost efficiency.
Conclusion: Making the Best Choice for Water Treatment
Choosing between water softening and deionization depends on specific water treatment goals, such as hardness removal versus total ion elimination. Water softening effectively reduces calcium and magnesium to prevent scale buildup, while deionization removes a broader spectrum of dissolved ions for high-purity applications. Understanding the intended use, water quality requirements, and cost considerations ensures selecting the most efficient solution for optimal water treatment results.
water softening vs deionization Infographic
 libmatt.com
libmatt.com