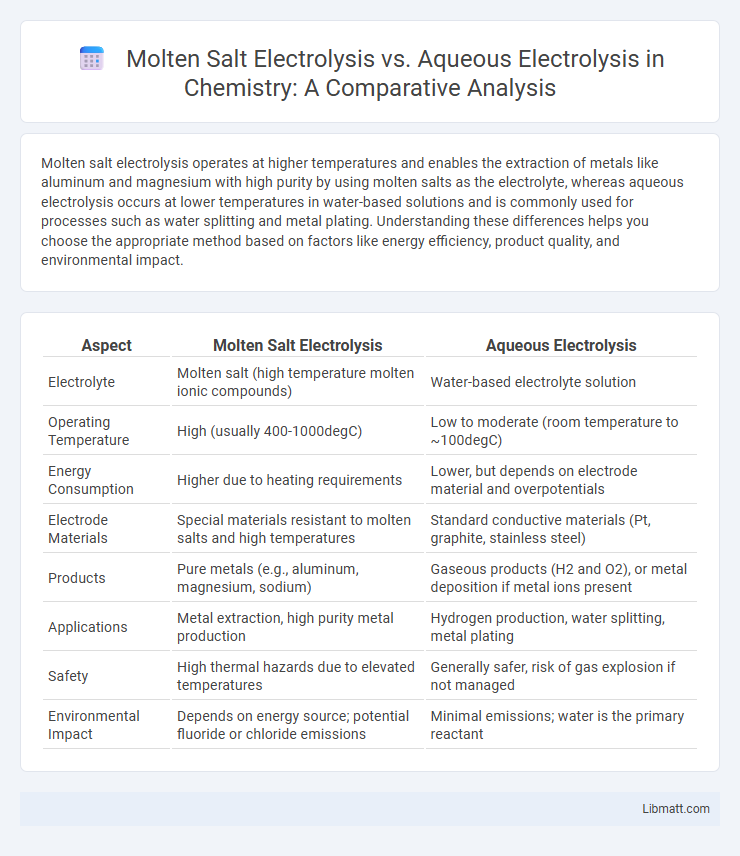
Molten salt electrolysis operates at higher temperatures and enables the extraction of metals like aluminum and magnesium with high purity by using molten salts as the electrolyte, whereas aqueous electrolysis occurs at lower temperatures in water-based solutions and is commonly used for processes such as water splitting and metal plating. Understanding these differences helps you choose the appropriate method based on factors like energy efficiency, product quality, and environmental impact.
Table of Comparison
| Aspect | Molten Salt Electrolysis | Aqueous Electrolysis |
|---|---|---|
| Electrolyte | Molten salt (high temperature molten ionic compounds) | Water-based electrolyte solution |
| Operating Temperature | High (usually 400-1000degC) | Low to moderate (room temperature to ~100degC) |
| Energy Consumption | Higher due to heating requirements | Lower, but depends on electrode material and overpotentials |
| Electrode Materials | Special materials resistant to molten salts and high temperatures | Standard conductive materials (Pt, graphite, stainless steel) |
| Products | Pure metals (e.g., aluminum, magnesium, sodium) | Gaseous products (H2 and O2), or metal deposition if metal ions present |
| Applications | Metal extraction, high purity metal production | Hydrogen production, water splitting, metal plating |
| Safety | High thermal hazards due to elevated temperatures | Generally safer, risk of gas explosion if not managed |
| Environmental Impact | Depends on energy source; potential fluoride or chloride emissions | Minimal emissions; water is the primary reactant |
Introduction to Electrolysis Methods
Molten salt electrolysis involves the decomposition of ionic compounds in a high-temperature molten state, enabling the extraction of metals such as aluminum and magnesium with high efficiency and purity. Aqueous electrolysis, on the other hand, uses water-based solutions to drive chemical reactions at lower temperatures, commonly applied in hydrogen production and electroplating processes. Both methods utilize electric current to induce redox reactions, but differ significantly in operational conditions, electrode materials, and product outputs.
Principles of Molten Salt Electrolysis
Molten salt electrolysis operates by dissolving ionic compounds in a high-temperature molten state, enabling the conduction of electricity through freely moving ions for metal extraction or chemical synthesis. Unlike aqueous electrolysis, which uses water-based solutions with limited electrochemical windows, molten salt electrolysis benefits from higher operational temperatures and a broader electrochemical range, minimizing side reactions such as hydrogen evolution. This process typically involves a robust inert electrode system and a carefully controlled atmosphere to maintain salt purity and prevent oxidation, making it ideal for producing reactive metals like aluminum, magnesium, and lithium.
Fundamentals of Aqueous Electrolysis
Aqueous electrolysis involves the decomposition of water into hydrogen and oxygen gases using an electric current passed through an electrolyte solution, typically containing acids, bases, or salts to enhance conductivity. This process relies on the movement of ions in the liquid phase, where water molecules are split at the electrodes by electron transfer reactions, with hydrogen evolving at the cathode and oxygen at the anode. Understanding your aqueous electrolysis system requires considering factors such as electrode material, electrolyte concentration, voltage applied, and temperature, as these influence efficiency and gas purity.
Key Differences in Electrolyte Composition
Molten salt electrolysis utilizes high-temperature ionic salts that remain liquid above 300degC, offering a non-aqueous, highly conductive medium for electrochemical reactions, whereas aqueous electrolysis involves water-based electrolytes containing dissolved ions. The absence of water in molten salt electrolysis prevents hydrogen evolution and allows the reduction of metal oxides and other compounds unstable in water. Consequently, molten salt electrolytes enable higher temperature operations and access to reactive species, contrasting with the relatively low-temperature, water-dependent environment in aqueous electrolysis.
Electrode Reactions: Molten Salt vs. Aqueous Systems
In molten salt electrolysis, electrode reactions involve the direct reduction or oxidation of ions in a high-temperature, anhydrous environment, typically producing pure metals or elemental gases without water interference. Aqueous electrolysis relies on water as the solvent, where electrode reactions include hydrogen or oxygen evolution alongside metal ion reduction, often complicated by competing side reactions like hydrogen evolution. The key difference lies in the electrolyte's physical state and ion availability, influencing reaction kinetics, product purity, and operational temperature ranges.
Temperature Requirements and Energy Efficiency
Molten salt electrolysis operates at high temperatures, typically between 700degC and 1000degC, enabling efficient ion mobility and reducing electrode polarization, which enhances overall energy efficiency. In contrast, aqueous electrolysis functions at much lower temperatures, around 25degC to 100degC, but suffers from increased energy consumption due to overpotentials and limited ionic conductivity of water-based electrolytes. The elevated temperature in molten salt electrolysis facilitates faster reaction kinetics and reduced electrical resistance, resulting in superior energy efficiency compared to traditional aqueous systems.
Industrial Applications and Scalability
Molten salt electrolysis offers high-temperature stability and efficient ion transport, making it ideal for large-scale metal extraction and industrial production of aluminum and magnesium. In contrast, aqueous electrolysis is widely used for hydrogen generation and wastewater treatment but faces scalability challenges due to lower current density and energy efficiency. Your choice between these methods depends on industrial application requirements and desired production volume scalability.
Product Purity and Yield Considerations
Molten salt electrolysis typically achieves higher product purity and yield due to its ability to operate at elevated temperatures, reducing contamination and side reactions common in aqueous electrolysis. The absence of water in molten salt systems prevents hydrogen evolution, thereby increasing metal deposition efficiency and overall yield. Your choice between these methods should consider the desired product purity and yield, as molten salt electrolysis is often preferred for high-purity metal extraction and industrial-scale production.
Environmental and Safety Aspects
Molten salt electrolysis operates at high temperatures, reducing water usage and minimizing hydrogen gas emissions, which lessens the risk of flammable gas explosions compared to aqueous electrolysis. Aqueous electrolysis involves handling corrosive electrolytes and produces hydrogen and oxygen gases that require careful ventilation to prevent fire hazards. Molten salt systems, while energy-intensive and requiring thermal insulation, often present lower environmental contamination risks due to the absence of liquid effluents common in aqueous processes.
Future Trends in Electrolysis Technologies
Molten salt electrolysis offers superior efficiency and higher operating temperatures, enabling enhanced hydrogen production compared to aqueous electrolysis, which is limited by water's electrochemical window. Future trends indicate a shift toward integrating renewable energy sources with molten salt systems to improve sustainability and lower costs. Your choice of electrolysis technology will increasingly depend on advancements in materials and system designs that optimize performance for large-scale clean energy applications.
Molten salt electrolysis vs aqueous electrolysis Infographic
 libmatt.com
libmatt.com