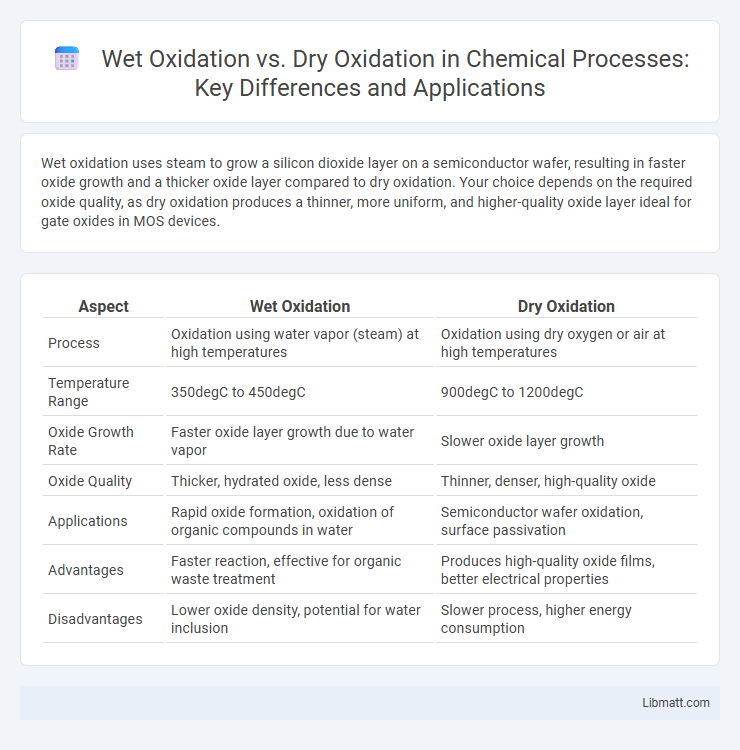
Wet oxidation uses steam to grow a silicon dioxide layer on a semiconductor wafer, resulting in faster oxide growth and a thicker oxide layer compared to dry oxidation. Your choice depends on the required oxide quality, as dry oxidation produces a thinner, more uniform, and higher-quality oxide layer ideal for gate oxides in MOS devices.
Table of Comparison
| Aspect | Wet Oxidation | Dry Oxidation |
|---|---|---|
| Process | Oxidation using water vapor (steam) at high temperatures | Oxidation using dry oxygen or air at high temperatures |
| Temperature Range | 350degC to 450degC | 900degC to 1200degC |
| Oxide Growth Rate | Faster oxide layer growth due to water vapor | Slower oxide layer growth |
| Oxide Quality | Thicker, hydrated oxide, less dense | Thinner, denser, high-quality oxide |
| Applications | Rapid oxide formation, oxidation of organic compounds in water | Semiconductor wafer oxidation, surface passivation |
| Advantages | Faster reaction, effective for organic waste treatment | Produces high-quality oxide films, better electrical properties |
| Disadvantages | Lower oxide density, potential for water inclusion | Slower process, higher energy consumption |
Introduction to Silicon Oxidation Techniques
Silicon oxidation techniques are essential in semiconductor manufacturing, with wet oxidation using steam to grow a silicon dioxide layer faster, resulting in thicker oxide films ideal for insulation and passivation. Dry oxidation, involving pure oxygen, produces thinner, high-quality oxide layers suitable for gate oxides in MOSFET devices due to its superior density and electrical properties. Understanding these methods helps optimize your device performance by selecting the appropriate oxide growth process based on thickness and quality requirements.
Overview of Wet Oxidation
Wet oxidation involves the use of water vapor or steam combined with oxygen at elevated temperatures to rapidly grow a thin oxide layer on semiconductor surfaces, enhancing silicon wafer passivation. This process typically occurs at temperatures between 800degC and 1000degC, providing faster oxide growth rates compared to dry oxidation due to increased oxidizing species diffusion. Your semiconductor device's performance benefits from wet oxidation's ability to form thicker oxides efficiently while maintaining desirable electrical insulation properties.
Overview of Dry Oxidation
Dry oxidation involves the reaction of silicon wafers with oxygen gas (O2) at high temperatures, typically between 900degC and 1200degC, to form a thin, dense layer of silicon dioxide (SiO2). This process produces high-quality oxide layers with superior electrical properties and better interface stability, making it ideal for gate oxides in MOS devices. Compared to wet oxidation, dry oxidation grows oxide layers more slowly but results in thinner, more uniform films essential for advanced semiconductor manufacturing.
Chemical Reactions Involved
Wet oxidation utilizes water vapor to introduce oxygen atoms during silicon dioxide layer formation, resulting in faster growth rates due to the diffusion of H2O molecules and subsequent reaction with silicon (Si + 2H2O - SiO2 + 2H2). Dry oxidation involves the direct reaction of molecular oxygen with silicon atoms (Si + O2 - SiO2) at elevated temperatures, producing denser and higher-quality oxide layers. These differing chemical reactions affect oxide thickness, density, and electrical properties important for semiconductor device fabrication.
Key Differences Between Wet and Dry Oxidation
Wet oxidation uses water vapor to grow silicon dioxide at higher rates and lower temperatures, resulting in thicker oxide layers, while dry oxidation employs pure oxygen to create thinner, denser oxide films with superior electrical properties. Wet oxidation is typically favored for producing thicker oxides due to faster growth but with inferior quality compared to dry oxidation, which is preferred for fine device structures requiring high-quality gate oxides. The choice between wet and dry oxidation impacts oxide thickness, growth rate, film quality, and electrical characteristics critical for semiconductor device performance.
Oxide Thickness and Growth Rates
Wet oxidation produces thicker silicon dioxide layers more rapidly due to the higher diffusion rate of water vapor compared to dry oxygen. Dry oxidation yields thinner, more uniform oxides with slower growth rates, ideal for gate oxides in MOSFETs requiring precise control. The growth rate in wet oxidation can be up to ten times faster than dry oxidation, significantly impacting process time and oxide quality.
Electrical Properties Comparison
Wet oxidation produces silicon dioxide layers with higher etch rates and lower density, resulting in inferior electrical properties such as reduced dielectric strength and higher leakage currents compared to dry oxidation. Dry oxidation yields denser, higher-quality oxide films with superior electrical isolation, lower interface trap density, and enhanced reliability for semiconductor devices. The choice between wet and dry oxidation significantly impacts gate oxide integrity, threshold voltage stability, and overall device performance in MOSFET fabrication.
Applications in Semiconductor Fabrication
Wet oxidation and dry oxidation play critical roles in semiconductor fabrication by forming silicon dioxide layers with varying characteristics. Wet oxidation, using steam, produces thicker oxide layers rapidly, making it ideal for field oxides and isolation regions, while dry oxidation yields thinner, high-quality oxides essential for gate dielectrics in MOS devices. Selecting between these processes depends on your device's required oxide thickness, uniformity, and electrical properties.
Advantages and Disadvantages
Wet oxidation offers faster oxidation rates and lower processing temperatures, making it ideal for forming thin oxide layers on silicon wafers with reduced thermal budget. However, it produces a less dense oxide layer with inferior electrical properties compared to dry oxidation, which yields high-quality, dense oxides essential for advanced semiconductor devices but requires longer processing times and higher temperatures. Understanding these differences helps you choose the appropriate oxidation method for optimizing device performance and manufacturing efficiency.
Choosing the Right Oxidation Method
Wet oxidation involves using steam to grow a silicon dioxide layer rapidly, producing a thicker oxide with faster processing times, ideal for applications requiring high oxidation rates and thicker films. Dry oxidation uses dry oxygen, resulting in a thinner, denser, and higher-quality oxide layer with better electrical properties, preferred for gate oxides in MOS devices. Selecting between wet and dry oxidation depends on the desired oxide thickness, film quality, and device performance requirements, with wet oxidation favoring speed and thickness, while dry oxidation emphasizes precision and reliability.
Wet oxidation vs dry oxidation Infographic
 libmatt.com
libmatt.com