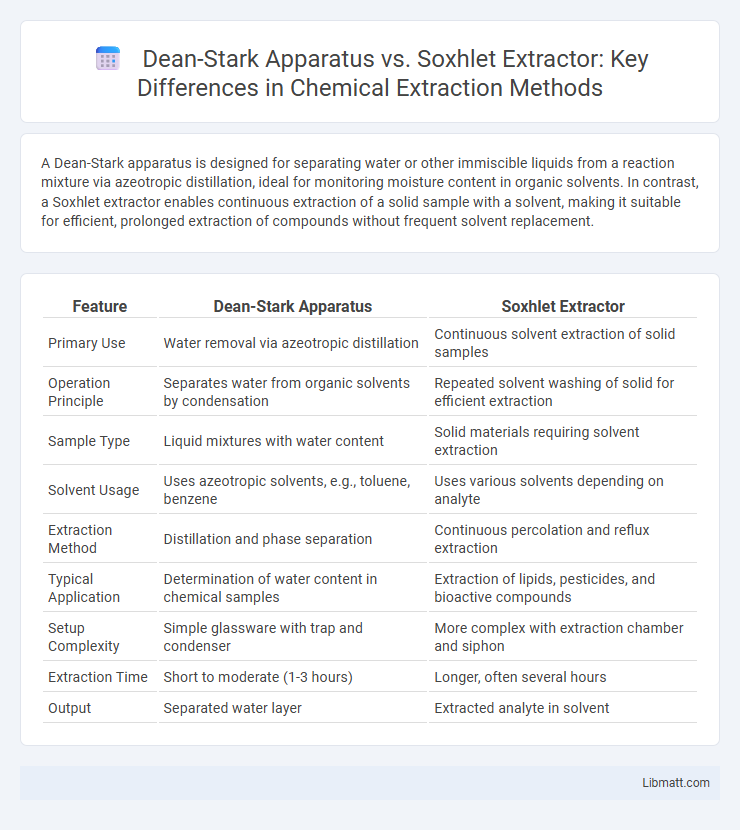
A Dean-Stark apparatus is designed for separating water or other immiscible liquids from a reaction mixture via azeotropic distillation, ideal for monitoring moisture content in organic solvents. In contrast, a Soxhlet extractor enables continuous extraction of a solid sample with a solvent, making it suitable for efficient, prolonged extraction of compounds without frequent solvent replacement.
Table of Comparison
| Feature | Dean-Stark Apparatus | Soxhlet Extractor |
|---|---|---|
| Primary Use | Water removal via azeotropic distillation | Continuous solvent extraction of solid samples |
| Operation Principle | Separates water from organic solvents by condensation | Repeated solvent washing of solid for efficient extraction |
| Sample Type | Liquid mixtures with water content | Solid materials requiring solvent extraction |
| Solvent Usage | Uses azeotropic solvents, e.g., toluene, benzene | Uses various solvents depending on analyte |
| Extraction Method | Distillation and phase separation | Continuous percolation and reflux extraction |
| Typical Application | Determination of water content in chemical samples | Extraction of lipids, pesticides, and bioactive compounds |
| Setup Complexity | Simple glassware with trap and condenser | More complex with extraction chamber and siphon |
| Extraction Time | Short to moderate (1-3 hours) | Longer, often several hours |
| Output | Separated water layer | Extracted analyte in solvent |
Overview of Dean-Stark Apparatus and Soxhlet Extractor
The Dean-Stark apparatus is a laboratory tool designed for the precise removal and quantification of water or other immiscible liquids from reaction mixtures via azeotropic distillation. The Soxhlet extractor, on the other hand, facilitates continuous solvent extraction of solid samples, enabling efficient and exhaustive compound recovery without the need for repeated manual solvent additions. You can select the Dean-Stark apparatus for moisture determination in chemical synthesis, while the Soxhlet extractor is ideal for extracting lipids or bioactive compounds from solid matrices.
Historical Background and Development
The Dean-Stark apparatus, developed by Ernest Dean and David Stark in the early 20th century, revolutionized moisture determination through its innovative liquid-liquid separation technique essential in organic synthesis and azeotropic distillations. The Soxhlet extractor, invented by Franz von Soxhlet in 1879, emerged as a pioneering device for continuous solvent extraction, significantly enhancing the efficiency of solid-liquid separation in chemical analysis and essential oil extraction. Your choice between these devices depends on the specific needs of quantitative liquid separation or exhaustive solid extraction in laboratory workflows.
Principle of Operation: Dean-Stark Apparatus
The Dean-Stark apparatus operates on the principle of azeotropic distillation to separate water from a reaction mixture by condensing the vapor and collecting the water in a graduated trap. This method efficiently quantifies the water content in liquid mixtures, especially in organic synthesis and esterification reactions. Unlike the Soxhlet extractor, which relies on solvent extraction and repeated washing, the Dean-Stark apparatus is specifically designed for continuous removal and measurement of water during reflux.
Principle of Operation: Soxhlet Extractor
The Soxhlet extractor operates on the principle of continuous solvent extraction, where a solvent repeatedly vaporizes, condenses, and washes over a solid sample to dissolve targeted compounds. This cyclic process ensures efficient extraction by allowing fresh solvent to contact the material multiple times without manual intervention. The apparatus is particularly effective for extracting lipids and other substances with limited solubility in the solvent used.
Key Applications in Analytical Chemistry
The Dean-Stark apparatus is primarily used for quantifying water content in samples through azeotropic distillation, essential in determining moisture levels in solvents, oils, and organic compounds. The Soxhlet extractor excels in continuous solvent extraction, ideal for isolating lipids, pesticides, and other analytes from solid matrices in environmental and food chemistry. Both instruments are crucial in analytical chemistry for sample preparation and precise quantification of target compounds in complex mixtures.
Comparative Efficiency and Yield
The Dean-Stark apparatus offers higher efficiency in separating water from organic solvents, making it ideal for moisture determination in chemical reactions, while the Soxhlet extractor excels in continuous extraction of solid samples, ensuring maximum yield of target compounds. Your choice depends on the specific application: use the Dean-Stark for precise water removal and the Soxhlet for exhaustive extraction of bioactive substances. Both devices optimize extraction processes, but the Soxhlet generally provides greater extraction yield due to prolonged solvent-sample contact.
Solvent and Sample Compatibility
The Dean-Stark apparatus is best suited for removing water from organic solvents and is compatible with samples that form azeotropes with water, making it ideal for moisture determination in various chemical reactions. The Soxhlet extractor is designed for continuous solvent extraction, working effectively with solid samples and solvents that dissolve target compounds without degrading them, ensuring thorough extraction of analytes like lipids or pesticides. While the Dean-Stark method requires immiscible solvents with water, Soxhlet extraction offers broader solvent compatibility, accommodating polar and non-polar solvents depending on the sample's chemical nature.
Advantages and Limitations
The Dean-Stark apparatus offers precise water or azeotrope separation from reaction mixtures, making it ideal for monitoring moisture content in synthetic chemistry with minimal solvent loss. Its limitation lies in slow separation rates and ineffectiveness with non-volatile solvents, restricting its use to specific solvent-water systems. The Soxhlet extractor excels in continuous solvent extraction of solid samples, providing efficient compound recovery over extended periods, but it demands large solvent volumes and longer extraction times, which may lead to thermal degradation of heat-sensitive analytes.
Safety and Environmental Considerations
The Dean-Stark apparatus minimizes solvent use by efficiently separating water from reaction mixtures, reducing chemical waste and lowering environmental impact compared to the Soxhlet extractor, which operates with continuous solvent cycling and higher consumption. Safety risks associated with the Dean-Stark include handling hot solvents in closed systems, necessitating proper ventilation to avoid vapor buildup, whereas the Soxhlet extractor presents increased hazards due to prolonged exposure to solvent vapors and potential overheating during extended reflux cycles. Both setups require appropriate laboratory precautions, but the Dean-Stark's shorter operation time and reduced solvent volume often translate to safer and more environmentally friendly organic extraction processes.
Choosing Between Dean-Stark and Soxhlet: Key Factors
Choosing between a Dean-Stark apparatus and Soxhlet extractor depends on the type of separation required and the nature of the sample. The Dean-Stark apparatus excels in removing water or other immiscible liquids from reaction mixtures via azeotropic distillation, making it ideal for moisture content determination. Soxhlet extractors are preferable for continuous solid-liquid extraction, efficiently isolating target compounds from solid matrices without the need for constant solvent replenishment.
Dean-Stark apparatus vs Soxhlet extractor Infographic
 libmatt.com
libmatt.com