A distillation column separates components based on differences in boiling points using vapor-liquid equilibrium, while an absorption column removes specific gases from a mixture by dissolving them into a liquid solvent. Your choice between the two depends on whether you need phase separation by volatility or selective mass transfer into a liquid.
Table of Comparison
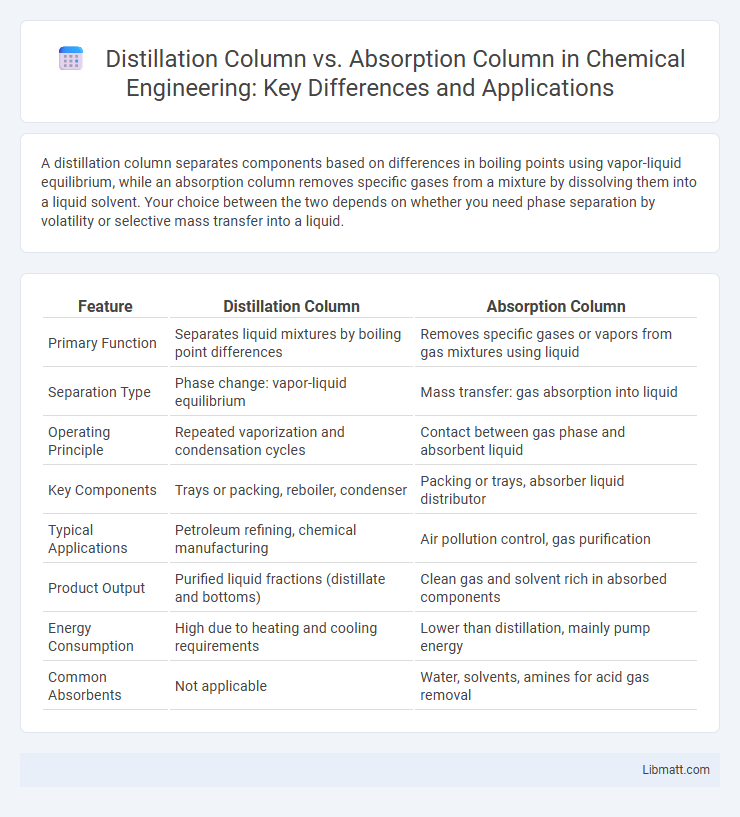
| Feature | Distillation Column | Absorption Column |
|---|---|---|
| Primary Function | Separates liquid mixtures by boiling point differences | Removes specific gases or vapors from gas mixtures using liquid |
| Separation Type | Phase change: vapor-liquid equilibrium | Mass transfer: gas absorption into liquid |
| Operating Principle | Repeated vaporization and condensation cycles | Contact between gas phase and absorbent liquid |
| Key Components | Trays or packing, reboiler, condenser | Packing or trays, absorber liquid distributor |
| Typical Applications | Petroleum refining, chemical manufacturing | Air pollution control, gas purification |
| Product Output | Purified liquid fractions (distillate and bottoms) | Clean gas and solvent rich in absorbed components |
| Energy Consumption | High due to heating and cooling requirements | Lower than distillation, mainly pump energy |
| Common Absorbents | Not applicable | Water, solvents, amines for acid gas removal |
Introduction to Separation Columns
Distillation columns separate liquid mixtures based on boiling point differences, using vapor-liquid equilibrium to achieve component separation efficiently. Absorption columns remove specific gas components by contacting the gas stream with a liquid solvent, leveraging solubility and mass transfer principles. Understanding these fundamental differences helps optimize Your process for energy use and product purity.
Overview of Distillation Columns
Distillation columns separate liquid mixtures into individual components by exploiting differences in boiling points through repeated vaporization and condensation cycles. You rely on precise temperature control and tray or packing design to enhance mass transfer efficiency within the column. These systems are critical in refining processes where high purity and component recovery are essential.
Overview of Absorption Columns
Absorption columns are designed to transfer specific components from a gas mixture into a liquid solvent through mass transfer processes, commonly used in gas purification and pollutant removal. These columns operate based on the differential solubility of gas components in the liquid phase, facilitating efficient separation by contacting the ascending gas with descending absorbent on trays or packing materials. Absorption technology is favored in industries such as chemical processing, natural gas treatment, and environmental control due to its effectiveness in capturing volatile organic compounds and acid gases.
Working Principles: Distillation vs Absorption
A distillation column separates liquid mixtures based on differences in boiling points through repeated vaporization and condensation stages, allowing components with lower boiling points to rise and those with higher boiling points to descend. An absorption column operates by transferring a specific gas component from a gas mixture into a liquid absorbent, relying on solubility and mass transfer principles rather than phase change. Your choice depends on whether separation relies primarily on volatility differences (distillation) or selective solubility and mass transfer (absorption).
Key Design Differences
Distillation columns separate components based on differences in volatility through vapor-liquid equilibrium, requiring trays or packing designed for vapor-liquid contact and precise temperature control stages. Absorption columns rely on mass transfer between gas and liquid phases to remove specific components, emphasizing solvent selection, liquid distribution, and contact efficiency rather than vapor-liquid equilibrium stages. Design differences include operating pressure ranges, with distillation often under vacuum or high pressure, while absorption typically occurs at near-atmospheric pressures for optimal solvent performance.
Typical Applications of Distillation Columns
Distillation columns are primarily used for separating liquid mixtures based on differences in boiling points, making them ideal for refining crude oil, producing ethanol, and separating chemical solvents. They efficiently separate volatile components in petrochemical industries, pharmaceutical manufacturing, and natural gas processing. Typical applications highlight their role in achieving high-purity products through fractional distillation of multicomponent mixtures.
Typical Applications of Absorption Columns
Absorption columns are typically used in gas treatment processes to selectively remove specific components, such as removing acid gases like CO2 or H2S from natural gas streams. You will find absorption columns commonly employed in industries like petrochemical refining, wastewater treatment, and air pollution control, where gas-liquid mass transfer is critical. These columns excel at capturing pollutants and recovering valuable solvents by facilitating intimate contact between the gaseous and liquid phases.
Advantages and Limitations of Each Column
Distillation columns offer high separation efficiency for mixtures with significant boiling point differences but require substantial energy input due to repeated vaporization-condensation cycles. Absorption columns excel in selectively removing specific components from gas streams using a liquid solvent, providing energy savings compared to distillation but are limited by solvent regeneration challenges and lower operational temperatures. Your choice depends on feed composition, energy costs, and desired purity, balancing these advantages and limitations to optimize process performance.
Factors Affecting Column Selection
Column selection between distillation and absorption primarily depends on the volatility difference of the components and the desired separation efficiency. Distillation columns are ideal for separating mixtures with significant volatility differences, while absorption columns are preferred when a specific component needs to be selectively removed using a solvent. Key factors affecting this choice include feed composition, operating costs, solvent availability, and temperature-pressure conditions.
Conclusion: Choosing the Right Column for Your Process
Selecting the appropriate separation column depends on the specific requirements of your process, such as the volatility of components and desired purity levels. Distillation columns excel at separating mixtures based on boiling points for volatile compounds, while absorption columns are ideal for removing specific gas components using a solvent. Evaluating factors like feed composition, operating conditions, and product specifications ensures the best choice between distillation and absorption columns for optimal efficiency and cost-effectiveness.
distillation column vs absorption column Infographic
 libmatt.com
libmatt.com