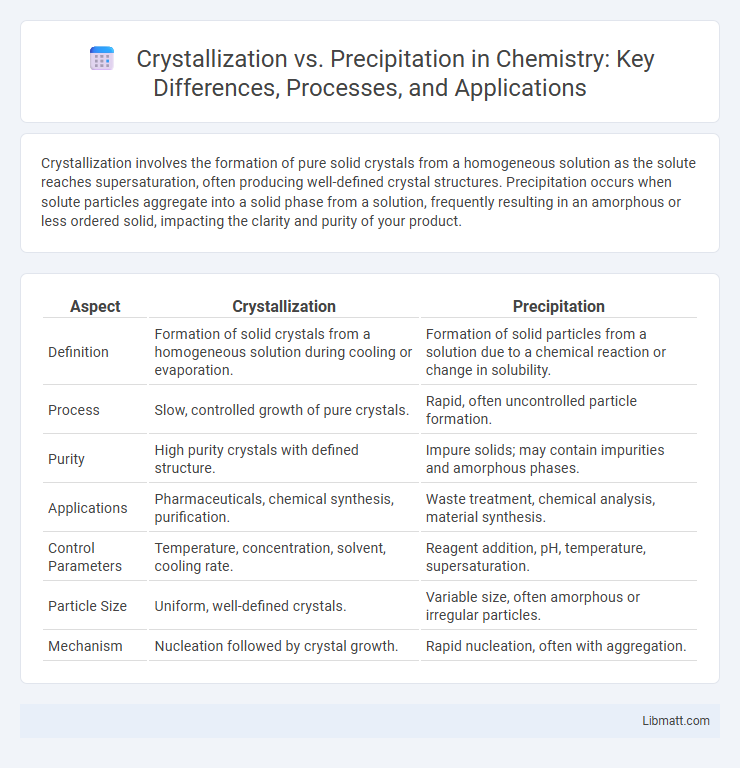
Crystallization involves the formation of pure solid crystals from a homogeneous solution as the solute reaches supersaturation, often producing well-defined crystal structures. Precipitation occurs when solute particles aggregate into a solid phase from a solution, frequently resulting in an amorphous or less ordered solid, impacting the clarity and purity of your product.
Table of Comparison
| Aspect | Crystallization | Precipitation |
|---|---|---|
| Definition | Formation of solid crystals from a homogeneous solution during cooling or evaporation. | Formation of solid particles from a solution due to a chemical reaction or change in solubility. |
| Process | Slow, controlled growth of pure crystals. | Rapid, often uncontrolled particle formation. |
| Purity | High purity crystals with defined structure. | Impure solids; may contain impurities and amorphous phases. |
| Applications | Pharmaceuticals, chemical synthesis, purification. | Waste treatment, chemical analysis, material synthesis. |
| Control Parameters | Temperature, concentration, solvent, cooling rate. | Reagent addition, pH, temperature, supersaturation. |
| Particle Size | Uniform, well-defined crystals. | Variable size, often amorphous or irregular particles. |
| Mechanism | Nucleation followed by crystal growth. | Rapid nucleation, often with aggregation. |
Introduction to Crystallization and Precipitation
Crystallization and precipitation are fundamental processes in chemistry where solid particles form from a solution, but crystallization results in highly ordered crystal lattices, while precipitation often produces amorphous or less-ordered solids. Crystallization is used to purify substances by controlling factors such as temperature and concentration to grow crystals, whereas precipitation occurs more rapidly and is commonly employed to separate compounds in analytical and industrial procedures. Understanding these differences helps optimize Your methods for material synthesis and analytical recovery.
Defining Crystallization
Crystallization is a process where a solid forms from a solution or melt, resulting in a well-ordered, structured lattice of molecules or ions. This controlled formation allows for the purification of substances by selectively forming crystals while impurities remain in the solution. Your understanding of crystallization helps differentiate it from precipitation, which usually involves rapid, uncontrolled solid formation without an organized crystal structure.
Understanding Precipitation
Precipitation is the process where dissolved substances in a solution form solid particles that settle out of the liquid, often due to changes in temperature, concentration, or chemical reactions. Unlike crystallization, which typically produces structured and pure solids, precipitation can result in amorphous or impure solids depending on the conditions. Understanding precipitation helps you control solution purities and optimize separation techniques in chemical and industrial processes.
Key Differences Between Crystallization and Precipitation
Crystallization involves the formation of highly ordered solid crystals from a solution, often resulting in pure and uniform crystals, while precipitation produces a solid that may be amorphous or less ordered. Crystallization typically occurs through controlled cooling or evaporation, enabling selective crystallite growth, whereas precipitation often results from a sudden chemical reaction or mixing of reagents. The purity and morphology of products differ significantly, with crystallization yielding well-defined structures and precipitation producing heterogeneous solids.
Mechanisms of Crystallization
Crystallization involves the formation of a highly ordered solid structure from a solution, melt, or gas phase through nucleation and crystal growth, driven by supersaturation. Nucleation initiates the process by forming small, stable clusters of molecules or ions, which then grow as additional particles attach to these nuclei. The mechanism depends on factors such as concentration, temperature, solvent properties, and impurities, influencing crystal size, shape, and purity.
Mechanisms of Precipitation
Precipitation occurs when dissolved ions or molecules in a solution exceed their solubility limit, causing solid particles to form rapidly and disperse throughout the liquid. This mechanism involves nucleation followed by growth, where supersaturation drives the transformation from dissolved species to a solid phase without the need for an orderly, crystalline structure. Your understanding of precipitation mechanisms is crucial for controlling processes in chemistry and material science, such as water treatment and pharmaceuticals.
Factors Influencing Crystallization
Crystallization depends on factors such as temperature, supersaturation level, and the presence of impurities, which affect crystal size and purity. Controlled cooling rates and solvent choice also play critical roles in promoting orderly crystal lattice formation. In contrast, precipitation primarily occurs from a rapid increase in concentration or chemical reaction, often resulting in amorphous or less structured solids.
Factors Affecting Precipitation
Precipitation is influenced by factors such as solute concentration, temperature, and the presence of impurities, which alter solubility and nucleation rates. Changes in pH and ionic strength can also impact ion interactions, promoting or inhibiting precipitate formation. Understanding these variables is essential for controlling the efficiency and purity of the resulting solid phase.
Applications of Crystallization and Precipitation
Crystallization is widely used in pharmaceuticals to purify compounds and control particle size for drug formulation, while precipitation plays a critical role in water treatment by removing contaminants and heavy metals. In chemical manufacturing, crystallization helps isolate pure products from a solution, whereas precipitation is essential for producing pigments and fertilizers by transforming dissolved ions into solid particles. Your choice between crystallization and precipitation depends on the desired purity and particle characteristics required in the specific industrial application.
Conclusion: Choosing the Right Process
Crystallization offers precise control over crystal size and purity, making it ideal for applications requiring high-quality solid formation. Precipitation is faster and suitable for rapid solid removal but may produce less uniform particles. Your choice depends on desired product characteristics, process speed, and operational complexity.
Crystallization vs precipitation Infographic
 libmatt.com
libmatt.com