Gradient elution involves gradually changing the solvent composition during the chromatographic run to improve the separation of complex mixtures, whereas isocratic elution maintains a constant solvent composition throughout the process. Your choice between these methods depends on the sample complexity and the need for resolution efficiency versus operational simplicity.
Table of Comparison
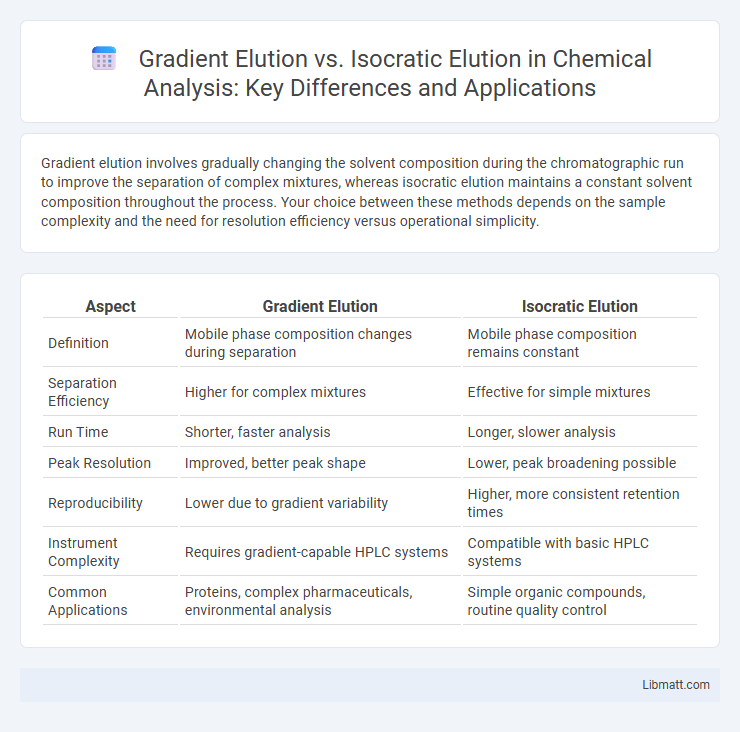
| Aspect | Gradient Elution | Isocratic Elution |
|---|---|---|
| Definition | Mobile phase composition changes during separation | Mobile phase composition remains constant |
| Separation Efficiency | Higher for complex mixtures | Effective for simple mixtures |
| Run Time | Shorter, faster analysis | Longer, slower analysis |
| Peak Resolution | Improved, better peak shape | Lower, peak broadening possible |
| Reproducibility | Lower due to gradient variability | Higher, more consistent retention times |
| Instrument Complexity | Requires gradient-capable HPLC systems | Compatible with basic HPLC systems |
| Common Applications | Proteins, complex pharmaceuticals, environmental analysis | Simple organic compounds, routine quality control |
Introduction to Chromatographic Elution Techniques
Gradient elution involves varying the composition of the mobile phase during the chromatographic run to enhance the separation of complex mixtures, while isocratic elution maintains a constant mobile phase composition throughout the analysis. Gradient elution improves resolution and reduces analysis time for samples with broad polarity ranges, making it ideal for complex or closely related compounds. Isocratic elution offers simplicity, reproducibility, and is well-suited for samples with components of similar polarity and retention times.
Defining Isocratic Elution: Principles and Applications
Isocratic elution in liquid chromatography maintains a constant mobile phase composition throughout the separation process, offering consistent retention times and simplified method development. This technique is particularly effective for analyzing samples with components of similar polarity or when reproducible results are essential in quality control. Your choice of isocratic elution supports reliable quantification and straightforward troubleshooting in routine analytical applications.
Understanding Gradient Elution: How It Works
Gradient elution involves gradually changing the solvent composition during liquid chromatography to enhance separation efficiency and resolution of complex mixtures. By increasing the proportion of the stronger solvent over time, compounds with varying affinities for the stationary phase elute sequentially, reducing analysis time and peak overlap. This technique contrasts with isocratic elution, which uses a constant solvent composition throughout the run, often leading to longer retention times for late-eluting analytes.
Key Differences Between Isocratic and Gradient Elution
Isocratic elution uses a single, constant solvent composition throughout the chromatographic run, providing simplicity and reproducibility ideal for analyzing compounds with similar retention times. Gradient elution gradually changes the solvent composition, enhancing separation efficiency for complex mixtures by improving peak resolution and reducing analysis time. Key differences include solvent composition stability in isocratic versus dynamic changes in gradient elution, impacting selectivity, sensitivity, and suitability for mixtures of varying polarity.
Advantages of Isocratic Elution
Isocratic elution offers the advantage of simplicity and reproducibility by maintaining a constant mobile phase composition throughout the chromatographic run. This approach reduces method development time and is ideal for separating compounds with similar retention characteristics, ensuring consistent retention times and peak shapes. Your analysis benefits from easier system equilibration and lower solvent consumption compared to gradient elution.
Benefits of Gradient Elution
Gradient elution offers enhanced separation efficiency by gradually changing the solvent composition, allowing better resolution of complex mixtures compared to isocratic elution. This technique increases sensitivity and reduces analysis time, making it ideal for samples with a wide range of polarities. Your chromatographic performance improves with gradient elution, especially when dealing with components that differ significantly in retention behavior.
Limitations of Isocratic Elution
Isocratic elution is limited by its inability to effectively separate complex mixtures with components of varying polarity, often leading to prolonged analysis times and broader peaks. This method struggles with sample retention time variability when analytes differ significantly in their affinity for the stationary phase. Your choice of elution strategy should consider these limitations, especially when high resolution and faster run times are critical.
Challenges with Gradient Elution
Gradient elution in liquid chromatography faces challenges including complex method development and the need for precise solvent mixing to maintain reproducibility and resolution. The dynamic change in solvent composition can cause baseline drift and difficulty in quantifying analytes with similar retention times. Equipment demands are higher, requiring pumps capable of delivering accurate gradient profiles, which increases operational cost and maintenance frequency.
Choosing the Right Elution Method for Your Analysis
Gradient elution enhances separation efficiency by gradually changing the mobile phase composition, ideal for complex mixtures with components of varying polarity, while isocratic elution maintains a constant solvent ratio, offering simplicity and reproducibility for samples with similar polarity compounds. Selecting the right elution method depends on analytical goals: gradient elution reduces run times and improves peak resolution in high-performance liquid chromatography (HPLC) when analyzing samples with a broad range of analytes, whereas isocratic elution provides consistent retention times and easier method validation for routine analysis. The choice impacts sensitivity, selectivity, and overall chromatographic performance, influencing method development and optimization in quantitative and qualitative studies.
Conclusion: Optimizing Chromatographic Performance
Optimizing chromatographic performance requires selecting the appropriate elution method based on the sample complexity and target analytes. Gradient elution enhances resolution and reduces analysis time by gradually changing the mobile phase composition, making it ideal for complex mixtures, while isocratic elution provides reproducible and straightforward separation for simpler samples. Your choice between gradient and isocratic elution directly impacts separation efficiency, peak shape, and method robustness in liquid chromatography.
gradient elution vs isocratic elution Infographic
 libmatt.com
libmatt.com