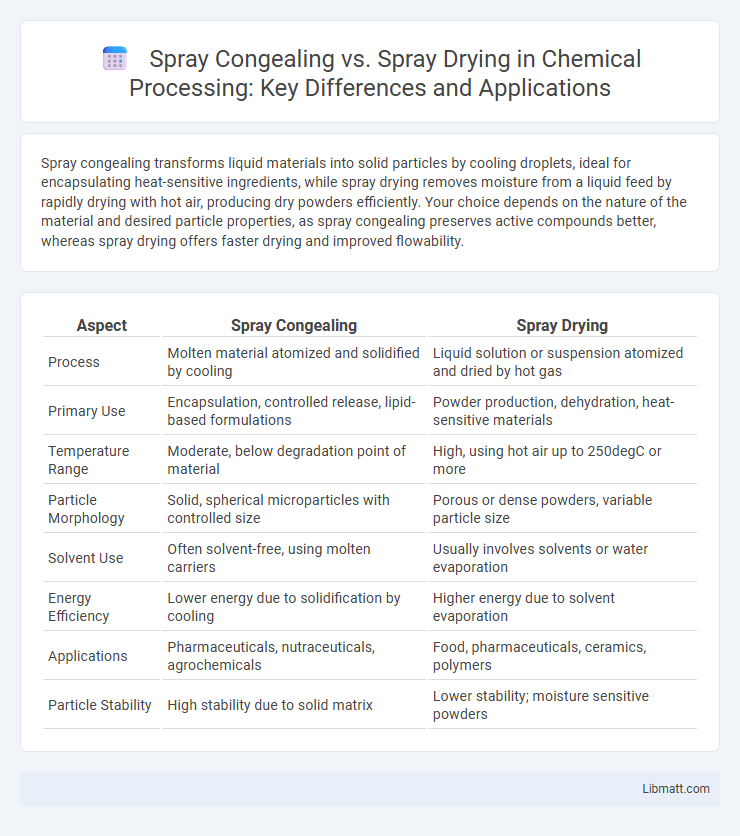
Spray congealing transforms liquid materials into solid particles by cooling droplets, ideal for encapsulating heat-sensitive ingredients, while spray drying removes moisture from a liquid feed by rapidly drying with hot air, producing dry powders efficiently. Your choice depends on the nature of the material and desired particle properties, as spray congealing preserves active compounds better, whereas spray drying offers faster drying and improved flowability.
Table of Comparison
| Aspect | Spray Congealing | Spray Drying |
|---|---|---|
| Process | Molten material atomized and solidified by cooling | Liquid solution or suspension atomized and dried by hot gas |
| Primary Use | Encapsulation, controlled release, lipid-based formulations | Powder production, dehydration, heat-sensitive materials |
| Temperature Range | Moderate, below degradation point of material | High, using hot air up to 250degC or more |
| Particle Morphology | Solid, spherical microparticles with controlled size | Porous or dense powders, variable particle size |
| Solvent Use | Often solvent-free, using molten carriers | Usually involves solvents or water evaporation |
| Energy Efficiency | Lower energy due to solidification by cooling | Higher energy due to solvent evaporation |
| Applications | Pharmaceuticals, nutraceuticals, agrochemicals | Food, pharmaceuticals, ceramics, polymers |
| Particle Stability | High stability due to solid matrix | Lower stability; moisture sensitive powders |
Introduction to Spray Congealing and Spray Drying
Spray congealing involves atomizing a molten material into a cooling chamber where droplets solidify upon contact with cooler air, commonly used for controlled-release pharmaceuticals and encapsulation. Spray drying transforms liquid feed into dry powder by spraying it into a hot gas environment, optimizing particle size and moisture content for food, pharmaceuticals, and chemical industries. Both techniques enhance product stability and bioavailability by tailoring particle morphology and size distribution.
Core Principles of Spray Congealing
Spray congealing involves melting a solid lipid or wax and atomizing it into a cooling chamber where droplets solidify upon contact with cold air, forming microparticles. This process preserves the thermal-sensitive active ingredients by avoiding high-temperature exposure common in spray drying, which uses hot air to remove moisture from liquid feed. Your choice between these methods depends on the desired particle morphology, thermal stability of the core material, and application requirements in pharmaceuticals or food industries.
Core Principles of Spray Drying
Spray drying involves atomizing a liquid feed into a hot drying medium, rapidly evaporating the solvent to form solid particles suspended in air. This process relies on the principles of heat and mass transfer, where droplets undergo rapid moisture removal, resulting in fine, uniform powders. Key parameters influencing spray drying efficiency include inlet temperature, feed rate, and atomization technique, ensuring controlled particle size and moisture content.
Key Differences Between Spray Congealing and Spray Drying
Spray congealing solidifies molten materials by cooling atomized droplets into fine solid particles, while spray drying removes moisture from liquid feed by rapidly drying atomized droplets with hot gas. Spray congealing is ideal for encapsulating heat-sensitive ingredients due to lower processing temperatures, whereas spray drying excels in producing dry powders from liquid suspensions or solutions quickly. Your choice between these techniques depends on the thermal sensitivity of your materials and the desired particle properties, such as density and morphology.
Equipment and Process Parameters
Spray congealing uses equipment with a heated atomizer to melt and atomize molten materials, while spray drying employs a nozzle or rotary atomizer to disperse liquid feed into hot drying air. Process parameters for spray congealing include temperature control to maintain the material in a molten state, atomization pressure, and cooling rate to solidify particles, whereas spray drying focuses on inlet and outlet air temperature, feed rate, and drying air flow to evaporate moisture efficiently. Understanding these differences in equipment and parameters helps optimize your particle characteristics for applications like pharmaceuticals or food ingredients.
Material Compatibility and Applications
Spray congealing is ideal for materials with low melting points, such as lipids and waxes, enabling encapsulation of heat-sensitive compounds in pharmaceuticals and food industries. Spray drying suits aqueous solutions or suspensions of heat-stable materials like proteins, enzymes, and dairy products, widely applied in food processing and biotechnology. Material compatibility dictates that spray congealing preserves bioactivity of thermolabile substances, whereas spray drying efficiently produces powdered forms for easier storage and transport.
Particle Morphology and Characteristics
Spray congealing produces solid lipid particles with smooth, spherical morphology and uniform size distribution due to controlled solidification of molten material, enhancing stability and controlled release properties. Spray drying typically results in porous, irregularly shaped particles with a broader size distribution caused by rapid solvent evaporation from liquid droplets. The dense and non-porous particles from spray congealing are advantageous for encapsulating heat-sensitive compounds, while spray drying particles offer high surface area suitable for enhanced solubility and dissolution rates.
Advantages and Limitations of Each Technique
Spray congealing offers advantages such as the ability to produce solid lipid microparticles with controlled drug release and improved stability, but it is limited by high viscosity feed materials and potential thermal degradation. Spray drying excels in rapid solvent removal to create dry powders with uniform particle size, yet it can result in lower encapsulation efficiency and is less suitable for heat-sensitive compounds. Both techniques require careful optimization of process parameters to balance particle morphology, drug loading, and stability tailored to specific pharmaceutical applications.
Industrial and Pharmaceutical Use Cases
Spray congealing is widely used in pharmaceuticals for controlled-release drug formulations and taste masking by solidifying molten materials into microparticles, enhancing bioavailability and stability. Spray drying is extensively applied in the food and pharmaceutical industries for producing powders with uniform particle size and preserving heat-sensitive ingredients through rapid solvent evaporation. Both technologies optimize drug delivery systems but differ in mechanism; spray congealing solidifies via cooling, while spray drying removes solvent by evaporation, making each suitable for specific industrial applications.
Choosing the Right Method: Decision Factors
Choosing the right method between spray congealing and spray drying depends on your formulation's thermal sensitivity, particle morphology requirements, and desired release profile. Spray congealing is ideal for encapsulating heat-sensitive active ingredients with a lipid matrix, enhancing controlled release and stability, while spray drying suits aqueous solutions needing rapid solvent evaporation and fine powder production. Consider your target particle size, solvent system, and scalability to optimize process efficiency and product performance.
spray congealing vs spray drying Infographic
 libmatt.com
libmatt.com